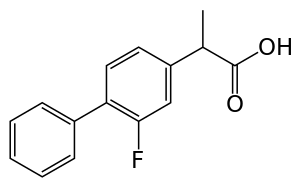
Flurbiprofen
 | |
| Names | |
|---|---|
| Trade names | Ansaid, Ocufen, Strepfen |
| Other names | (±)-2-fluoro-α-methyl-(1,1'-biphenyl)-4-acetic acid |
IUPAC name
| |
| Clinical data | |
| Drug class | Nonsteroidal anti-inflammatory drugs (NSAIDs)[1] |
| Main uses | By mouth: Rheumatoid arthritis, ankylosing spondylitis[1] Eye drop: Decrease pupil constriction, inflammation of the eye[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Systemic: Monograph Eye: Monograph |
| MedlinePlus | a687005 |
| Pharmacokinetics | |
| Protein binding | > 99% |
| Metabolism | Liver (CYP2C9) |
| Elimination half-life | 4.7-5.7 hours |
| Excretion | Kidney |
| Chemical and physical data | |
| Formula | C15H13FO2 |
| Molar mass | 244.265 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| Melting point | 117 °C (243 °F) |
SMILES
| |
InChI
| |
Flurbiprofen is a nonsteroidal anti-inflammatory drugs (NSAIDs) used to treat pain and inflammation.[1][3] By mouth it is used to treat painful periods, rheumatoid arthritis, and ankylosing spondylitis.[1][3] As an eye drop it is used to decrease pupil constriction during eye surgery and inflammation of the eye after surgery.[2]
Common side effects when taken by mouth include swelling, heart burn, gastrointestinal bleeding, nausea, liver problems, and anxiety.[1] Common side effects when used as an eye drop include burning, dry eyes, and large pupils.[2] Other side effects may include anaphylaxis and heart problems.[1] Use in the second half of pregnancy may harm the baby.[1] It works by blocking COX1 and COX2.[1]
Flurbiprofen was patented in 1964 and approved for medical use in 1987.[4] It became available as a generic medicaion in 1994.[5] In the United States 100 tablets of 100 mg costs about 66 USD as of 2021.[6] This amount in the United Kingdom costs about £92.[3]
Medical uses
Dosage
It is taken by mouth as 100 mg two to three times per day.[1]
Side effects
In October 2020, the U.S. Food and Drug Administration (FDA) required the drug label to be updated for all nonsteroidal anti-inflammatory medications to describe the risk of kidney problems in unborn babies that result in low amniotic fluid.[7][8] They recommend avoiding NSAIDs in pregnant women at 20 weeks or later in pregnancy.[7][8]
History
Flurbiprofen was patented in 1964 by Boots UK and approved for medical use in 1987.[4]
Society and culture
Cost
This medication has a cost in the U.S. of $18 (USD) for 15 tablets (100 mg).[9]
.svg.png.webp) Flurbiprofen costs (US)
Flurbiprofen costs (US).svg.png.webp) Flurbiprofen prescriptions (US)
Flurbiprofen prescriptions (US)
Brand names
As of 2016 the drug was available worldwide as drops for ophthalmic use and as tablets, both in various strengths, under many brand names which include: Acustop Cataplasma, Adofeed, Anazin, Anflupin, Anorcid, Ansaid, Antadys, Antafen, Antipain, Baenazin, Benactiv, Biprofin, Biprotec, Bro-Z, Brufen, Brufoz, Cebutid, Clinadol, Coryfin, Dispain, Edolfene, Eyeflur, Falken, Fiera, Flu Ro Fen, Flubifix, Flufen, Flugalin, Flupe, Flur di fen, Fluractive, Fluran, Flurbi Pap, Flurbic, Flurbiprofen, Flurbiprofène, Flurbiprofeno, Flurflex, Flurofen, Fluroptic, Fo Bi Pu Luo Fun, Forphen, Fortine, Froben, Frolix, Fubifen, Fubiprofen, Fubofen, Fukon, Fulruban, Furofen, Kai Fen, Kavoflog, Kotton, Lefenine, Majezik, Maprofen, Maxaljin, Maximus, Meiprofen, Neliacan, Nibelon, Nirolex Gola, Ocufen, Ocuflur, Optifen, Orofaringeo, Painil, Profen, Projezik, Ropion, Sigmaprofen, Stayban, Strefen, Strepfen, Strepflam, Strepsils (various formulations), Sulan, Tie Shr Shu, TransAct, Upnon, Urbifen, Yakuban, Zepolas, Zeralgo, Zero-P, and Zeton.[10]
References
- 1 2 3 4 5 6 7 8 9 "Flurbiprofen Monograph for Professionals". Drugs.com. Archived from the original on 28 August 2021. Retrieved 14 December 2021.
- 1 2 3 "Flurbiprofen (EENT) Monograph for Professionals". Drugs.com. Archived from the original on 28 January 2021. Retrieved 14 December 2021.
- 1 2 3 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1186. ISBN 978-0857114105.
- 1 2 Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 520. ISBN 9783527607495. Archived from the original on 2020-08-06. Retrieved 2020-10-17.
- ↑ Approved Drug Products with Therapeutic Equivalence Evaluations (PDF) (36th ed.). FDA. 2014. p. 158. Archived (PDF) from the original on 2016-11-15. Retrieved 2016-11-14.
- ↑ "Flurbiprofen Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 28 August 2021. Retrieved 14 December 2021.
- 1 2 "FDA Warns that Using a Type of Pain and Fever Medication in Second Half of Pregnancy Could Lead to Complications". U.S. Food and Drug Administration (FDA) (Press release). 15 October 2020. Archived from the original on 16 October 2020. Retrieved 15 October 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 "NSAIDs may cause rare kidney problems in unborn babies". U.S. Food and Drug Administration. 21 July 2017. Archived from the original on 17 October 2020. Retrieved 15 October 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Flurbiprofen Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 28 August 2021. Retrieved 30 March 2021.
- ↑ "Flurbiprofen - International Brand Names". Drugs.com. Archived from the original on 15 November 2016. Retrieved 14 November 2016.
External links
| Identifiers: |
|---|
- Dean L (2019). "Flurbiprofen Therapy and CYP2C9 Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 30742399. Bookshelf ID: NBK537365. Archived from the original on 2020-10-26. Retrieved 2020-02-05.