Lacosamide
 | |
| Names | |
|---|---|
| Trade names | Vimpat |
| Other names | (2R)-2-(acetylamino)-N-benzyl-3-methoxypropanamide |
IUPAC name
| |
| Clinical data | |
| Drug class | Anticonvulsant[1] |
| Main uses | Epilepsy, diabetic neuropathic pain[1][2] |
| Side effects | Dizziness, headache, nausea, tiredness, tremor, diarrhea[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth, intravenous |
| Typical dose | 50 to 200 mg BID[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a609028 |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | High |
| Elimination half-life | 13 hours |
| Excretion | Kidney |
| Chemical and physical data | |
| Formula | C13H18N2O3 |
| Molar mass | 250.298 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Lacosamide, sold under the brand name Vimpat among others, is a medication used to treat epilepsy and diabetic neuropathic pain.[1][2] It is taken by mouth or injection into a vein.[1] It may be taken by itself or with other medications.[1]
Common side effects include dizziness, headache, nausea, tiredness, tremor, and diarrhea.[1] Other side effects may include syncope, arrhythmias including atrial fibrillation, poor coordination, and suicide.[1] There are concerns that use in pregnancy may harm the baby.[1] How it works is not entirely unclear, but it is beleived to affect sodium channels in the brain.[2]
Lacosamide was approved for medical use in the United States and Europe in 2008.[1][2] In the United States a dose of 100 mg twice per day costs about 1,000 USD per month as of 2021.[3] In Canada this amount costs about 200 CAD.[4] In the United Kingdom this amount costs the NHS about £95.[5]
Medical uses
Lacosamide is used to treat epilepsy and neuropathic pain.[1][2]
Seizures
Lacosamide has been used for partial-onset seizures and generalized tonic clonic seizures.[2] It was found to reduce seizure frequency when given in addition to other antiepileptics, at doses of 400 and 600 milligrams a day.[6]
Clinical trials are currently underway for the use of lacosamide as monotherapy for partial-onset seizures.[7] There is no evidence that lacosamide provides additional value over current antiepileptic drugs (AEDs) for the treatment of partial-onset seizures, but it may offer a safety advantage.[8]
Neuropathy
In a smaller trial of diabetic neuropathy, lacosamide improved pain.[9] Further evidence is needed to determine usefulness.[1]
Dosage
Lacosamide is initially prescribed by mouth at 50 mg twice per day, with a total dose of 100 mg/day. The dosing can be increased by 100 mg/day following a twice-daily dose up to a total dose of 200–400 mg/day. Clinical trials showed that a dose of 600 mg/day was not more effective than a dose of 400 mg/day, but resulted in more adverse reactions. Lacosamide is administered orally through film-coated tablets of 50 mg (pink), 100 mg (dark yellow), 150 mg (salmon), and 200 mg (blue). It can also be administered by injection at a concentration of 200 mg/20 mL or by oral solution at a concentration of 10 mg/mL.[10]
Contraindications
The FDA has assigned lacosamide to pregnancy category C. Animal studies have reported incidences of fetal mortality and growth deficit. Lacosamide has not been tested during human pregnancy, and should be administered with caution. In addition, it has not been determined whether the excretion of lacosamide occurs in breast milk.[11]
Side effects
Lacosamide was generally well tolerated in adult patients with partial-onset seizures.[12] The side-effects most commonly leading to discontinuation were dizziness, ataxia, diplopia (double vision), nystagmus, nausea, vertigo and drowsiness. These adverse reactions were observed in at least 10% of patients.[10] Less common side-effects include tremors, blurred vision, vomiting and headache.
Gastrointestinal
Central nervous system
Dizziness was the most common treatment-related adverse event. Other CNS effects are headache, drowsiness, blurred vision, involuntary movements, memory problems, diplopia (double vision), trembling or shaking of the hands, unsteadiness, ataxia.
Psychiatric
Panic attacks; agitation or restlessness; irritability and aggression, anxiety, or depression; suicidality; insomnia and mania; altered mood; false and unusual sense of well-being. Lacosamide appears to have a low incidence of psychiatric side effects with psychosis reported in only 0.3% of patients.[13]
Cardiovascular

Allergies
Warnings
Suicidal behavior and ideation have been observed as early as one week after starting treatment with lacosamide, and is an adverse reaction from use of most AEDs. In clinical trials with a medial treatment duration of 12 weeks, the incidence of suicidal ideation was 0.43% among 27,863 patients as opposed to 0.24% among 16,029 placebo-treated patients. Suicidal behavior was observed in 1 of every 530 patients treated.[13]
Pregnancy
In a study conducted to assess the teratogenic potential of AEDs in the zebrafish embryo, the teratogenicity index of lacosamide was found to be higher than that of lamotrigine, levetiracetam, and ethosuximide. Lacosamide administration resulted in different malformations in the neonatal zebrafish depending on dosage.[15]
Overdose
There is no known antidote in the event of an overdose.
Pharmacology
Pharmacodynamics
Lacosamide is a functionalized amino acid that produces activity in the maximal electroshock seizure (MES) test, that, like some other antiepileptic drugs (AEDs), are believed to act through voltage-gated sodium channels.[16] Lacosamide enhances the slow inactivation of voltage-gated sodium channels without affecting the fast inactivation of voltage-gated sodium channels. This inactivation prevents the channel from opening, helping end the action potential. Many antiepileptic drugs, like carbamazepine or lamotrigine, slow the recovery from inactivation and hence reduce the ability of neurons to fire action potentials. Inactivation only occurs in neurons firing action potentials; this means that drugs that modulate fast inactivation selectively reduce the firing in active cells. Slow inactivation is similar but does not produce complete blockade of voltage gated sodium channels, with both activation and inactivation occurring over hundreds of milliseconds or more. Lacosamide makes this inactivation happen at less depolarized membrane potentials. This means that lacosamide only affects neurons which are depolarized or active for long periods of time, typical of neurons at the focus of epilepsy.[17] Lacosamide administration results in the inhibition of repetitive neuronal firing, the stabilization of hyperexcitable neuronal membranes, and the reduction of long-term channel availability, but does not affect physiological function.[18] Lacosamide has a dual mechanism of action. It also modulates collapsin response mediator protein 2 (CRMP-2), preventing the formation of abnormal neuronal connections in the brain.[19]
Lacosamide does not affect AMPA, kainate, NMDA, GABAA, GABAB or a variety of dopaminergic, serotonergic, adrenergic, muscarinic or cannabinoid receptors and does not block potassium or calcium currents.[20] Lacosamide does not modulate the reuptake of neurotransmitters including norepinephrine, dopamine, and serotonin.[21] In addition, it does not inhibit GABA transaminase.[22]
Pharmacokinetics
When administered orally in healthy individuals, lacosamide is rapidly absorbed from the gastrointestinal tract. Little of the drug is lost via the first pass effect, and thus has an oral bioavailability of nearly 100%.[23] In adults, lacosamide demonstrates a low plasma protein binding of <15%, which reduces the potential for interaction with other drugs. Lacosamide is at its highest concentration in blood plasma approximately 1 to 4 hours after oral administration. Lacosamide has a half life of about 12–16 hours, which remains unchanged if the patients is also taking enzyme inducers. Consequently, the drug is administered twice per day at 12-hour intervals. Lacosamide is excreted renally, with 95% of the drug eliminated in the urine.[24] 40% of the compound remains unchanged from its original structure, while the rest of the elimination product consists of metabolites of lacosamide. Just 0.5% of the drug is eliminated in the feces.[25] The major metabolic pathway of lacosamide is CYP2C9, CY2C19, and CYP3A4-mediated demethylation.[26]
The dose-response curve for lacosamide is linear and proportional for oral doses of up to 800 mg and intravenous doses of up to 300 mg.[27] Lacosamide has low potential for drug-drug interactions, and no pharmacokinetic interactions have been found to occur with other (AEDs) that act on sodium channels.[8] A study on the binding of lacosamide to CRMP-2 in Xenopus oocytes showed both competitive and specific binding. Lacosamide has a Kd value just under 5μM and a Bmax of about 200 pM/mg.[28] The volume of distribution (Vd) of lacosamide in plasma is 0.6 L/kg, which is close to the total volume of water. Lacosamide is ampiphilic and is thus hydrophilic while also lipophilic enough to cross the blood-brain barrier.[29]
Chemistry
Lacosamide is a powdery, white to light yellow crystalline compound. The chemical name of lacosamide is (R)-2-acetamido-N-benzyl-3-methoxypropionamide and the systemic name is N2-Acetyl-N-benzyl-O-methyl-D-serinamide.[21][30] Lacosamide is a functionalized amino acid molecule that has high solubility in water and DMSO, with a solubility of 20.1 mg/mL in phosphate-buffered saline (PBS, pH 7.5, 25 °C).[21][31] The molecule has six rotatable bonds and one aromatic ring. Lacosamide melts at 143-144 °C and boils at 536.447 °C at a pressure of 760 mmHg.[25][32]
Synthesis
The following three-step synthesis of lacosamide was proposed in 1996.
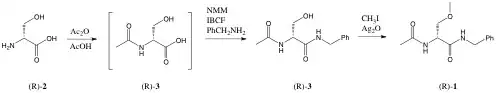
(R)-2-amino-3-hydroxypropanoic acid is treated with acetic anhydride and acetic acid. The product is treated first with N-methylmorpholine, isobutyl chloroformate, and benzylamine, next with methyl iodide and silver oxide, forming lacosamide.[33]
More efficient routes to synthesis have been proposed in recent years, including the following.[34]

History
Lacosamide was discovered by Dr. Harold Kohn, Dr. Shridhar Andurkar, and colleagues at the University of Houston in 1996.[33][36] They hypothesized that modified amino acids may be therapeutically useful in the treatment of epilepsy. A few hundred such molecules were synthesized over several years and these were tested phenotypically in an epilepsy disease model performed in rats. N-benzyl-2-acetamido-3-methoxypropionamide was found to be highly efficacious in this model, with the biological activity traced specifically to its R enantiomer.[33] This compound was to become Lacosamide after being licensed by Schwarz Pharma, which completed its pre-clinical and early clinical development. After its purchase of Schwarz Pharma in 2006, UCB completed the clinical development program and obtained marketing approval for Lacosamide. Its precise mechanism of action was unknown at the time of approval, and the exact amino acid targets involved remain uncertain to this day.[16]
The U.S. Food and Drug Administration (FDA) accepted UCB's New Drug Application for lacosamide as of November 29, 2007, beginning the approval process for the drug.[37][38] UCB also filed for marketing approval in the European Union (EU); the European Medicines Agency accepted the marketing application for review in May 2007.[37][39]
The drug was approved in the EU on September 3, 2008.[40] It was approved in the US on October 29, 2008.[41] Lacosamide release was delayed owing to an objection about its placement into schedule V of the Controlled Substances Act. The FDA issued their final rule of placement into Schedule V on June 22, 2009.[42]
Society and culture
Generic names
Lacosamide is the INN. It was formerly known as erlosamide, harkeroside, SPM-927, or ADD-234037.
Brand names
Lacosamide is marketed under the brand name Vimpat.
In Pakistan, it is marketed by The Searle Company Limited as Lacolit.
Research
Studies are underway for the use in fibromyalgia.[13]
In preclinical trials, the effect of lacosamide administration on animal models of epilepsy was tested using the Frings audiogenic seizures (AGS)-susceptible mouse model of seizure activity with an effective dose (ED50) of 0.63 mg/kg, i.p.[43] The effect of lacosamide was also assessed using the MES test to detect inhibition of seizure spread.[44][45] Lacosamide administration was successful in preventing the spread of seizures induced by MES in mice (ED50 = 4.5 mg/kg, i.p.) and rats (ED50 = 3.9 mg/kg, p.o.).[43] In preclinical trials, administration of lacosamide in combination with other AEDs resulted in synergistic anticonvulsant effects. Lacosamide produced effects in animal models of essential tremor, tardive dyskinesia, schizophrenia, and anxiety.[46] Preclinical trials found the S-stereoisomer to be less potent than the R-stereoisomer in the treatment of seizures.[47]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 "Lacosamide Monograph for Professionals". Drugs.com. Archived from the original on 10 August 2020. Retrieved 20 November 2021.
- 1 2 3 4 5 6 7 "Vimpat". Archived from the original on 1 August 2021. Retrieved 20 November 2021.
- ↑ "Vimpat Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 20 January 2021. Retrieved 20 November 2021.
- ↑ "Lacosamide" (PDF). CADTH. 2011. Archived (PDF) from the original on 20 October 2020. Retrieved 20 November 2021.
- ↑ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 334. ISBN 978-0857114105.
- ↑ Ben-Menachem E, Biton V, Jatuzis D, Abou-Khalil B, Doty P, Rudd GD (2007). "Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures". Epilepsia. 48 (7): 1308–17. doi:10.1111/j.1528-1167.2007.01188.x. PMID 17635557. S2CID 25986031.
- ↑ Doty, P; Hebert, D; Mathy, FX; Byrnes, W; Zackheim, J; Simontacchi, K (Jul 2013). "Development of lacosamide for the treatment of partial-onset seizures". Annals of the New York Academy of Sciences. 1291 (1): 56–68. Bibcode:2013NYASA1291...56D. doi:10.1111/nyas.12213. PMC 3759704. PMID 23859801.
- 1 2 "Therapeutic Class Review" (PDF). RegenceRx. Archived from the original (PDF) on 7 April 2014. Retrieved 2 April 2014.
- ↑ Rauck RL, Shaibani A, Biton V, Simpson J, Koch B (2007). "Lacosamide in painful diabetic peripheral neuropathy: a phase 2 double-blind placebo-controlled study". Clin J Pain. 23 (2): 150–8. doi:10.1097/01.ajp.0000210957.39621.b2. PMID 17237664. S2CID 6651958.
- 1 2 "Highlights of Prescribing Information" (PDF). Vimpat. Archived from the original (PDF) on 17 June 2012. Retrieved 2 April 2014.
- ↑ "Lacosamide Pregnancy and Breastfeeding Warnings". Drugs.com. Archived from the original on 2 April 2019. Retrieved 2 April 2014.
- ↑ Cross SA, Curran MP (2009). "Lacosamide". Drugs. 69 (4): 449–459. doi:10.2165/00003495-200969040-00005. PMID 19323588. Archived from the original on 2011-10-08. Retrieved 2021-09-22.
- 1 2 3 Halford, J. J.; Lapointe, M. (2009). "Clinical Perspectives on Lacosamide". Epilepsy Currents. 9 (1): 1–9. doi:10.1111/j.1535-7511.2008.01273.x. PMC 2668106. PMID 19396339.
- ↑ Vimpat Side Effects Center http://www.rxlist.com/vimpat-side-effects-drug-center.html Archived 2016-08-21 at the Wayback Machine
- ↑ Lee, SH; Kang, JW; Lin, T; Lee, JE; Jin, DI (2013). "Teratogenic potential of antiepileptic drugs in the zebrafish model". BioMed Research International. 2013: 1–6. doi:10.1155/2013/726478. PMC 3845484. PMID 24324971.
- 1 2 Michael A. Rogawski; Azita Tofighy; H. Steve White; Alain Matagne; Christian Wolff (2015). "Current understanding of the mechanism of action of the antiepileptic drug lacosamide". Epilepsy Research. 110: 189–205. doi:10.1016/j.eplepsyres.2014.11.021. PMID 25616473. S2CID 36351106. Archived from the original on 2019-05-12. Retrieved 2021-09-22.
- ↑ Errington AC, Stöhr T, Heers C, Lees G (January 2008). "The investigational anticonvulsant lacosamide selectively enhances slow inactivation of voltage-gated sodium channels". Molecular Pharmacology. 73 (1): 157–69. doi:10.1124/mol.107.039867. PMID 17940193. S2CID 8318846. Archived from the original on 2021-10-31. Retrieved 2021-09-22.
- ↑ Doty, P; Hebert D; Mathy FX; Byrnes W; Zackheim J; Simontacchi K (2013). "Development of lacosamide for the treatment of partial-onset seizures". Ann N Y Acad Sci. 1291 (1): 56–68. Bibcode:2013NYASA1291...56D. doi:10.1111/nyas.12213. PMC 3759704. PMID 23859801.
- ↑ "SCHWARZ PHARMA Highlights the Results of 13 Lacosamide Data Presentations at North American Regional Epilepsy Congress in San Diego". Schwarz Pharma. 5 December 1996. Archived from the original on 25 June 2016. Retrieved 2 April 2014.
- ↑ Errington AC, Coyne L, Stöhr T, Selve N, Lees G (June 2006). "Seeking a mechanism of action for the novel anticonvulsant lacosamide". Neuropharmacology. 50 (8): 1016–29. doi:10.1016/j.neuropharm.2006.02.002. PMID 16620882. S2CID 19491712.
- 1 2 3 Beyreuther, BK; Freitag, J; Heers, C; Krebsfänger, N; Scharfenecker, U; Stöhr, T (Spring 2007). "Lacosamide: a review of preclinical properties". CNS Drug Reviews. 13 (1): 21–42. doi:10.1111/j.1527-3458.2007.00001.x. PMC 6494128. PMID 17461888.
- ↑ Errington, AC; Coyne, L; Stöhr, T; Selve, N; Lees, G (Jun 2006). "Seeking a mechanism of action for the novel anticonvulsant lacosamide". Neuropharmacology. 50 (8): 1016–29. doi:10.1016/j.neuropharm.2006.02.002. PMID 16620882. S2CID 19491712.
- ↑ Hovinga, CA (2003). "SPM-927 (Schwarz Pharma)". IDrugs. 6 (5): 479–85. PMID 12789603.
- ↑ Italiano, D; Perucca E (2013). "Clinical pharmacokinetics of new-generation antiepileptic drugs at the extremes of age: an update". Clin Pharmacokinet. 52 (8): 627–45. doi:10.1007/s40262-013-0067-4. PMID 23640503. S2CID 33169643.
- 1 2 "Lacosamide". DrugBank. Archived from the original on 25 March 2014. Retrieved 2 April 2014.
- ↑ Abou-Khalil, BW (2009). "Lacosamide: what can be expected from the next new antiepileptic drug?". Epilepsy Curr. 9 (5): 133–4. doi:10.1111/j.1535-7511.2009.01317.x. PMC 2759042. PMID 19826503.
- ↑ Bialer, M; Johannessen SI; Kupferberg HJ; Levy RH; Perucca E; Tomson T (2004). "Progress report on new antiepileptic drugs: a summary of the Seventh Eilat Conference (EILAT VII)". Epilepsy Res. 61 (1–3): 1–48. doi:10.1016/j.eplepsyres.2004.07.010. PMID 15570674. S2CID 1154454.
- ↑ "Method for identifying CRMP modulators". Archived from the original on 11 June 2014. Retrieved 2 April 2014.
- ↑ Stoht, T; Kupferberg HJ; Stables JP; Choi D; Kohn H; Walton N; White HS (2007). "Lacosamide, a novel anti-convulsant drug, shows efficacy with a wide safety margin in rodent models for epilepsy". Epilepsy Res. 74 (2–3): 147–54. doi:10.1016/j.eplepsyres.2007.03.004. PMID 17433624. S2CID 23678213.
- ↑ "Lacosamide". ChemSpider. Archived from the original on 7 April 2014. Retrieved 2 April 2014.
- ↑ Biton, V; Rosenfeld, WE; Whitesides, J; Fountain, NB; Vaiciene, N; Rudd, GD (Mar 2008). "Intravenous lacosamide as replacement for oral lacosamide in patients with partial-onset seizures". Epilepsia. 49 (3): 418–24. doi:10.1111/j.1528-1167.2007.01317.x. PMID 17888078. S2CID 32471914.
- ↑ Kellinghaus, C (2009). "Lacosamide as treatment for partial epilepsy: mechanisms of action, pharmacology, effects, and safety". Therapeutics and Clinical Risk Management. 5: 757–66. doi:10.2147/tcrm.s5189. PMC 2754090. PMID 19816574.
- 1 2 3 Choi, D; Stables, JP; Kohn, H (Apr 26, 1996). "Synthesis and anticonvulsant activities of N-Benzyl-2-acetamidopropionamide derivatives". Journal of Medicinal Chemistry. 39 (9): 1907–16. doi:10.1021/jm9508705. PMID 8627614.
- ↑ Morieux, P; Stables, JP; Kohn, H (Oct 1, 2008). "Synthesis and anticonvulsant activities of N-benzyl-(2R)-2-acetamido-3-oxysubstituted propionamide derivatives". Bioorganic & Medicinal Chemistry. 16 (19): 8968–75. doi:10.1016/j.bmc.2008.08.055. PMC 2701728. PMID 18789868.
- ↑ McIntyre, J.A.; Castañer, J.; Martín, L. (2004). "Lacosamide". Drugs of the Future. 29 (10): 992. doi:10.1358/dof.2004.029.10.848936. ISSN 0377-8282.
- ↑ "Anticonvulsant enantiomeric amino acid derivatives". google.com. Archived from the original on 2021-10-31. Retrieved 2021-09-22.
- 1 2 "UCB Announces FDA Filing for lacosamide in the Treatment of Diabetic Neuropathic Pain" (Press release). UCB. 2007-11-29. Archived from the original on 2008-09-25. Retrieved 2007-11-29.
- ↑ "UCB Announces FDA Filing for lacosamide in the Treatment of Partial Onset Seizures in Adults with Epilepsy" (Press release). UCB. 2007-11-29. Archived from the original on 2008-09-25. Retrieved 2007-11-29.
- ↑ Wan, Yuet (August 17, 2007). "Marketing application for lacosamide (Vimpat) filed in EU for treatment of diabetic neuropathic pain". PharmaTimes through the UK National electronic Library for Medicines. Archived from the original on 2012-02-09. Retrieved 2007-11-30.
- ↑ "Vimpat Approved in Europe" (Press release). UCB. 2008-09-03. Archived from the original on 2008-09-19. Retrieved 2008-09-17.
- ↑ "UCB's Vimpat approved by U.S. FDA as adjunctive therapy for partial onset seizures in adults" (Press release). UCB. 2008-10-29. Archived from the original on 2008-11-14. Retrieved 2008-11-25.
- ↑ "FDA places lacosamide in Schedule V" (Press release). FDA. 2009-06-22. Archived from the original on 2011-07-08. Retrieved 2009-06-28.
- 1 2 Beyreuther, BK; Freitag J; Heers C; Krebsfanger N; Scharfenecker U; Stohr T (2007). "Lacosamide: a review of preclinical properties". CNS Drug Rev. 13 (1): 21–42. doi:10.1111/j.1527-3458.2007.00001.x. PMC 6494128. PMID 17461888.
- ↑ Borowicz, KK; Gaisor M; Kleinrok Z; Czuczwar SJ (1997). "Influence of isradipine, niguldipine and dantrolene on the anticonvulsive action of conventional antiepileptics in mice". Eur J Pharmacol. 323 (1): 45–51. doi:10.1016/s0014-2999(97)00020-4. PMID 9105875.
- ↑ Swinyard, EA; Brown WC; Godman LS (1952). "Comparative assays of antiepileptic drugs in mice and rats". J Pharmacol Exp Ther. 106 (3): 319–20. PMID 13000628.
- ↑ "SCHWARZ PHARMA Highlights the Results of 13 Lacosamide Data Presentations at North American Regional Epilepsy Congress in San Diego". Schwarz Pharma. 5 December 2006. Retrieved 2 April 2014.
- ↑ LeTiran, A; Stables, JP; Kohn, H (Oct 2001). "Functionalized amino acid anticonvulsants: synthesis and pharmacological evaluation of conformationally restricted analogues". Bioorganic & Medicinal Chemistry. 9 (10): 2693–708. doi:10.1016/s0968-0896(01)00204-8. PMID 11557357.
External links
- Dean L (2018). "Lacosamide Therapy and CYP2C19 Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 29671994. Bookshelf ID: NBK493589. Archived from the original on 2020-10-26. Retrieved 2021-09-22.
| External sites: |
|
|---|---|
| Identifiers: |
|