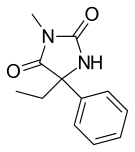
Mephenytoin
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a611020 |
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Metabolism | CYP2C19 |
| Elimination half-life | 7 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.012 |
| Chemical and physical data | |
| Formula | C12H14N2O2 |
| Molar mass | 218.256 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Mephenytoin (marketed as Mesantoin by Novartis) is a hydantoin, used as an anticonvulsant. It was introduced approximately 10 years after phenytoin, in the late 1940s. The significant metabolite of mephenytoin is nirvanol (5-ethyl-5-phenylhydantoin), which was the first hydantoin (briefly used as a hypnotic). However, nirvanol is quite toxic and mephenytoin was only considered after other less toxic anticonvulsants had failed. It can cause potentially fatal blood dyscrasia in 1% of patients.
Mephenytoin is no longer available in the US or the UK. It is still studied largely because of its interesting hydroxylation polymorphism.
References
- Shorvon SD, Fish DR, Perucca E, Dodson WE, eds. (2004). The Treatment of Epilepsy. Blackwell Publishing. ISBN 0-632-06046-8.
- Resor SR (1991). The Medical Treatment of Epilepsy. Marcel Dekker. ISBN 0-8247-8549-5.
- "Mephenytoin". The Comparative Toxicogenomics Database.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.