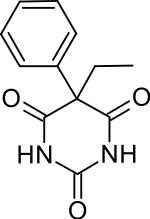
Phenobarbital
 | |
 | |
| Names | |
|---|---|
| Trade names | Luminal, others |
IUPAC name
| |
| Clinical data | |
| Drug class | Barbiturate[1] |
| Main uses | Status epilepticus, epilepsy[2][3] |
| Side effects | Decreased level of consciousness, decreased effort to breathe, abuse[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Dependence risk | Low[4] |
| Pregnancy category |
|
| Routes of use | By mouth (PO), rectal (PR), by injection (IM, IV) |
| Onset of action | Within 5 min (IV) and 30 min (PO)[1] |
| Duration of action | 4 hrs[1] to 2 days[5] |
| Defined daily dose | 100 mg[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682007 |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | >95% |
| Protein binding | 20 to 45% |
| Metabolism | Liver (mostly CYP2C19) |
| Elimination half-life | 53 to 118 hours |
| Excretion | Kidney and fecal |
| Chemical and physical data | |
| Formula | C12H12N2O3 |
| Molar mass | 232.239 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Phenobarbital, also known as phenobarbitone or phenobarb, is a medication of the barbiturate type.[1] It is recommended for the treatment of certain types of epilepsy in developing countries.[3] In the developed world, it is commonly used to treat seizures in young children,[6] while other medications are generally used in older children and adults.[7] It may be used intravenously, injected into a muscle, or taken by mouth.[1] The injectable form may be used to treat status epilepticus.[1] Phenobarbital is occasionally used to treat trouble sleeping, anxiety, and drug withdrawal and to help with surgery.[1] It usually begins working within five minutes when used intravenously and half an hour when administered by mouth.[1] Its effects last for between four hours and two days.[1][5]
Side effects include a decreased level of consciousness along with a decreased effort to breathe.[1] There is concern about both abuse and withdrawal following long-term use.[1] It may also increase the risk of suicide.[1] Use during pregnancy may cause harm to the baby.[1][8] If used during breastfeeding it may result in drowsiness in the baby.[9] A lower dose is recommended in those with poor liver or kidney function, as well as elderly people.[1] Phenobarbital, like other barbiturates works by increasing the activity of the inhibitory neurotransmitter GABA.[1]
Phenobarbital was discovered in 1912 and is the oldest still commonly used anti-seizure medication.[10][11] It is on the World Health Organization's List of Essential Medicines.[12] It is the least expensive anti-seizure medication at around US$5 a year in the developing world.[13] In the United States it generally costs less than 20 USD per month as of 2021.[14] Access, however, may be difficult as some countries label it as a controlled drug.[13]
Medical uses
Phenobarbital is used in the treatment of all types of seizures, except absence seizures.[15][16] It is no less effective at seizure control than phenytoin, however phenobarbital is not as well tolerated.[17] Phenobarbital may provide a clinical advantage over carbamazepine for treating partial onset seizures. Carbamazepine may provide a clinical advantage over phenobarbital for generalized onset tonic-clonic seizures.[18] Its very long active half-life (53–118 hours) means for some people doses do not have to be taken every day, particularly once the dose has been stabilized over a period of several weeks or months, and seizures are effectively controlled.
The first-line drugs for treatment of status epilepticus are benzodiazepines, such as lorazepam or diazepam. If these fail, then phenytoin may be used, with phenobarbital being an alternative in the US, but used only third-line in the UK.[19] Failing that, the only treatment is anaesthesia in intensive care.[16][20] The World Health Organization (WHO) gives phenobarbital a first-line recommendation in the developing world and it is commonly used there.[3][21]
Phenobarbital is the first-line choice for the treatment of neonatal seizures.[22][23][24] Concerns that neonatal seizures in themselves could be harmful make most physicians treat them aggressively. No reliable evidence, though, supports this approach.[25]
Phenobarbital is sometimes used for alcohol detoxification and benzodiazepine detoxification for its sedative and anti-convulsant properties. The benzodiazepines chlordiazepoxide (Librium) and oxazepam (Serax) have largely replaced phenobarbital for detoxification.[26]
While phenobarbital has been used for insomnia, such use is not recommended due to the risk of addiction and other side affects.[27]
Other uses
Phenobarbital properties can effectively reduce tremors and seizures associated with abrupt withdrawal from benzodiazepines.
Phenobarbital is a cytochrome P450 inducer, and is used to reduce the toxicity of some drugs.
Phenobarbital is occasionally prescribed in low doses to aid in the conjugation of bilirubin in people with Crigler–Najjar syndrome, type II,[28] or in patients with Gilbert's syndrome.[29]
Phenobarbital can also be used to relieve cyclic vomiting syndrome symptoms.
Phenobarbital is a commonly used agent in high purity and dosage for lethal injection of "death row" criminals.
In infants suspected of neonatal biliary atresia, phenobarbital is used in preparation for a 99mTc-IDA hepatobiliary (HIDA; hepatobiliary 99mTc-iminodiacetic acid) study that differentiates atresia from hepatitis or cholestasis.
Phenobarbital is used as a secondary agent to treat newborns with neonatal abstinence syndrome, a condition of withdrawal symptoms from exposure to opioid drugs in utero.
In massive doses, phenobarbital is prescribed to terminally ill patients to allow them to end their life through physician-assisted suicide.[30]
Like other barbiturates, phenobarbital can be used recreationally,[31] but this is reported to be relatively infrequent.[32]
Dosage
The defined daily dose for phenobarbital is 100 mg.[2] For adults with status epilepticus the initial dose is 20 mg/kg at a rate of 50 to 100 mg/min by injection.[33] A further dose of 5 to 10 mg/kg after 10 minutes may be used if not effective.[33] Ongoing maintenance is with 50 to 100 mg twice to three times per day.[33]
In children for status epilepticus 20 mg/kg over 10 minutes is used with maintenance at 2 to 3 mg/kg/day in either one or two doses.[33]
Side effects
Sedation and hypnosis are the principal side effects (occasionally, they are also the intended effects) of phenobarbital. Central nervous system effects, such as dizziness, nystagmus and ataxia, are also common. In older people, it may cause excitement and confusion, while in children, it may result in paradoxical hyperactivity.[34]
Phenobarbital is a cytochrome P450 hepatic enzyme inducer. It binds transcription factor receptors that activate cytochrome P450 transcription, thereby increasing its amount and thus its activity. Due to this higher amount of CYP450, drugs that are metabolized by the CYP450 enzyme system will have decreased effectiveness. This is because the increased CYP450 activity increases the clearance of the drug, reducing the amount of time they have to work.[35] It may decrease the effectiveness of birth control pills.[36]
Caution is to be used with children. Among anti-convulsant drugs, behavioural disturbances occur most frequently with clonazepam and phenobarbital.[37]
Contraindications include acute intermittent porphyria, hypersensitivity to any barbiturate, prior dependence on barbiturates, severe respiratory insufficiency (as with chronic obstructive pulmonary disease), and severe liver failure.[34]
Pregnancy and breastfeeding
The seriousness of untreated status epilepticus is greater than the risks of phenobarbital during pregnancy and breastfeeding.[36]
Overdose
Phenobarbital causes a depression of the body's systems, mainly the central and peripheral nervous systems. Thus, the main characteristic of phenobarbital overdose is a "slowing" of bodily functions, including decreased consciousness (even coma), bradycardia, bradypnea, hypothermia, and hypotension (in massive overdoses). Overdose may also lead to pulmonary edema and acute renal failure as a result of shock, and can result in death.
The electroencephalogram (EEG) of a person with phenobarbital overdose may show a marked decrease in electrical activity, to the point of mimicking brain death. This is due to profound depression of the central nervous system, and is usually reversible.[38]
Treatment of phenobarbital overdose is supportive, and mainly consists of the maintenance of airway patency (through endotracheal intubation and mechanical ventilation), correction of bradycardia and hypotension (with intravenous fluids and vasopressors, if necessary), and removal of as much drug as possible from the body. Depending on how much time has elapsed since ingestion of the drug, this may be accomplished through gastric lavage (stomach pumping) or use of activated charcoal. Hemodialysis is effective in removing phenobarbital from the body, and may reduce its half-life by up to 90%.[38] No specific antidote for barbiturate poisoning is available.[39]
Mechanism of action
Through its action on GABAA receptors, phenobarbital increases the flow of chloride ions into the neuron which decreases the excitability of the post-synaptic neuron. Hyperpolarizing this post-synaptic membrane leads to a decrease in the general excitatory aspects of the post-synaptic neuron. By making it harder to depolarize the neuron, the threshold for the action potential of the post-synaptic neuron will be increased. Phenobarbital stimulates GABA to accomplish this hyperpolarization.[40] Direct blockade of excitatory glutamate signaling is also believed to contribute to the hypnotic/anticonvulsant effect that is observed with the barbiturates.[41]
Pharmacokinetics
Phenobarbital has an oral bioavailability of about 90%. Peak plasma concentrations (Cmax) are reached eight to 12 hours after oral administration. It is one of the longest-acting barbiturates available – it remains in the body for a very long time (half-life of two to seven days) and has very low protein binding (20 to 45%). Phenobarbital is metabolized by the liver, mainly through hydroxylation and glucuronidation, and induces many isozymes of the cytochrome P450 system. Cytochrome P450 2B6 (CYP2B6) is specifically induced by phenobarbital via the CAR/RXR nuclear receptor heterodimer. It is excreted primarily by the kidneys.[42]
History
The first barbiturate drug, barbital, was synthesized in 1902 by German chemists Emil Fischer and Joseph von Mering and was first marketed as Veronal by Friedr. Bayer et comp. By 1904, several related drugs, including phenobarbital, had been synthesized by Fischer. Phenobarbital was brought to market in 1912 by the drug company Bayer as the brand Luminal. It remained a commonly prescribed sedative and hypnotic until the introduction of benzodiazepines in the 1960s.[43]
Phenobarbital's soporific, sedative and hypnotic properties were well known in 1912, but it was not yet known to be an effective anti-convulsant. The young doctor Alfred Hauptmann[44] gave it to his epilepsy patients as a tranquilizer and discovered their seizures were susceptible to the drug. Hauptmann performed a careful study of his patients over an extended period. Most of these patients were using the only effective drug then available, bromide, which had terrible side effects and limited efficacy. On phenobarbital, their epilepsy was much improved: The worst patients suffered fewer and lighter seizures and some patients became seizure-free. In addition, they improved physically and mentally as bromides were removed from their regimen. Patients who had been institutionalised due to the severity of their epilepsy were able to leave and, in some cases, resume employment. Hauptmann dismissed concerns that its effectiveness in stalling seizures could lead to patients suffering a build-up that needed to be "discharged". As he expected, withdrawal of the drug led to an increase in seizure frequency – it was not a cure. The drug was quickly adopted as the first widely effective anti-convulsant, though World War I delayed its introduction in the U.S.[45]
In 1939, a German family asked Adolf Hitler to have their disabled son killed; the five-month-old boy was given a lethal dose of Luminal after Hitler sent his own doctor to examine him. A few days later 15 psychiatrists were summoned to Hitler's Chancellery and directed to commence a clandestine euthanasia program.[46][47]
In 1940, at a clinic in Ansbach, Germany, around 50 intellectually disabled children were injected with Luminal and killed that way. A plaque was erected in their memory in 1988 in the local hospital at Feuchtwanger Strasse 38, although a newer plaque does not mention that patients were killed using barbiturates on site.[48][49] Luminal was used in the Nazi children's euthanasia program until at least 1943.[50][51]
Phenobarbital was used to treat neonatal jaundice by increasing liver metabolism and thus lowering bilirubin levels. In the 1950s, phototherapy was discovered, and became the standard treatment.[52]
Phenobarbital was used for over 25 years as prophylaxis in the treatment of febrile seizures.[53] Although an effective treatment in preventing recurrent febrile seizures, it had no positive effect on patient outcome or risk of developing epilepsy. The treatment of simple febrile seizures with anticonvulsant prophylaxis is no longer recommended.[54][55]
Society and culture
Names
Synthesis
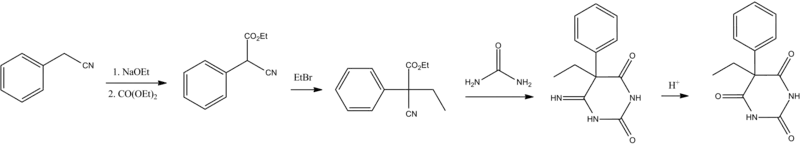
Barbiturate drugs are obtained via condensation reactions between a derivative of diethyl malonate and urea in the presence of a strong base.[56] The synthesis of phenobarbital uses this common approach as well but differs in the way in which this malonate derivative is obtained. The reason for this difference is due to the fact that aryl halides do not typically undergo nucleophilic substitution in Malonic ester synthesis in the same way as aliphatic organosulfates or halocarbons do.[57] To overcome this lack of chemical reactivity two dominant synthetic approaches using benzyl cyanide as a starting material have been developed:
The first of these methods consists of a Pinner reaction of benzyl cyanide, giving phenylacetic acid ethyl ester.[58] Subsequently, this ester undergoes cross Claisen condensation using diethyl oxalate, giving diethyl ester of phenyloxobutandioic acid. Upon heating this intermediate easily loses carbon monoxide, yielding diethyl phenylmalonate.[59] Malonic ester synthesis using ethyl bromide leads to the formation of α-phenyl-α-ethylmalonic ester. Finally a condensation reaction with urea gives phenobarbital.[56]
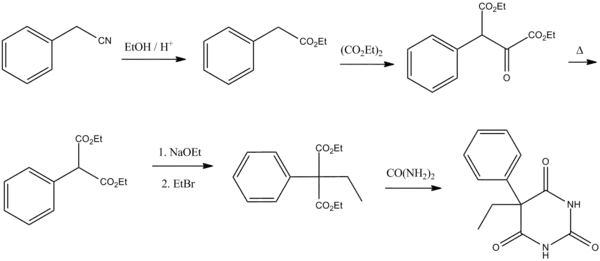
The second approach utilizes diethyl carbonate in the presence of a strong base to give α-phenylcyanoacetic ester.[60][61] Alkylation of this ester using ethyl bromide proceeds via a nitrile anion intermediate to give the α-phenyl-α-ethylcyanoacetic ester.[62] This product is then further converted into the 4-iminoderivative upon condensation with urea. Finally acidic hydrolysis of the resulting product gives phenobarbital.[63]
Regulation
The level of regulation includes Schedule IV non-narcotic (depressant) (ACSCN 2285) in the United States under the Controlled Substances Act 1970—but along with a few other barbiturates and at least one benzodiazepine, and codeine, dionine, or dihydrocodeine at low concentrations, it also has exempt prescription and had at least one exempt OTC combination drug now more tightly regulated for its ephedrine content.[64] The phenobarbitone/phenobarbital exists in subtherapeutic doses which add up to an effective dose to counter the overstimulation and possible seizures from a deliberate overdose in ephedrine tablets for asthma, which are now regulated at the federal and state level as: a restricted OTC medicine and/or watched precursor, uncontrolled but watched/restricted prescription drug & watched precursor, a Schedule II, III, IV, or V prescription-only controlled substance & watched precursor, or a Schedule V (which also has possible regulations at the county/parish, town, city, or district as well aside from the fact that the pharmacist can also choose not to sell it, and photo ID and signing a register is required) exempt Non-Narcotic restricted/watched OTC medicine.[65]
Notable overdoses
British veterinarian Donald Sinclair, better known as the character Siegfried Farnon in the "All Creatures Great and Small" book series by James Herriot, committed suicide at the age of 84 by injecting himself with an overdose of phenobarbital. Activist Abbie Hoffman also committed suicide by consuming phenobarbital, combined with alcohol, on April 12, 1989; the residue of around 150 pills was found in his body at autopsy.[66] Also dying from an overdose was British actress Phyllis Barry in 1954 and actress/model Margaux Hemingway in 1996.
The Japanese officers aboard the German submarine U-234 killed themselves with phenobarbital while the German crew members were on their way to the US to surrender (but before Japan had surrendered).
Thirty-nine members of the Heaven's Gate UFO religious group committed mass suicide in March 1997 by drinking a lethal dose of phenobarbital and vodka "and then lay down to die" hoping to enter an alien spacecraft.[67]
A mysterious woman, known as the Isdal Woman, was found dead in Bergen, Norway, on 29 November 1970. Her death was caused by some combination of burns, phenobarbital, and carbon monoxide poisoning; many theories about her death have been posited, and it is believed that she may have been a spy.[68]
Veterinary uses
Phenobarbital is one of the initial drugs of choice to treat epilepsy in dogs, and is the initial drug of choice to treat epilepsy in cats.[69]
It is also used to treat feline hyperesthesia syndrome in cats when anti-obsessional therapies prove ineffective.[70]
It may also be used to treat seizures in horses when benzodiazepine treatment has failed or is contraindicated.[71]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 "Phenobarbital". The American Society of Health-System Pharmacists. Archived from the original on 2015-09-06. Retrieved Aug 14, 2015.
- 1 2 3 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 27 October 2020. Retrieved 4 September 2020.
- 1 2 3 Ilangaratne, NB; Mannakkara, NN; Bell, GS; Sander, JW (Dec 1, 2012). "Phenobarbital: missing in action". Bulletin of the World Health Organization. 90 (12): 871–871A. doi:10.2471/BLT.12.113183. PMC 3524964. PMID 23284189.
- ↑ Bassert, Joanna M. (2017). McCurnin's Clinical Textbook for Veterinary Technicians - E-Book. Elsevier Health Sciences. p. 955. ISBN 9780323496407. Archived from the original on 2020-08-18. Retrieved 2020-05-09.
- 1 2 Marx, John A. (2010). Rosen's emergency medicine : concepts and clinical practice (7 ed.). Philadelphia: Mosby/Elsevier. p. 1352. ISBN 978-0-323-05472-0. Archived from the original on 2016-03-05.
- ↑ Brodie, MJ; Kwan, P (December 2012). "Current position of phenobarbital in epilepsy and its future". Epilepsia. 53 Suppl 8: 40–6. doi:10.1111/epi.12027. PMID 23205961.
- ↑ "The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care NICE guidelines [CG137]". National Institute for Health and Care Excellence. January 2012.
{{cite web}}: Missing or empty|url=(help) - ↑ "Prescribing medicines in pregnancy database". Australian Government. 3 March 2014. Archived from the original on 8 April 2014. Retrieved 22 April 2014.
- ↑ "Phenobarbital use while Breastfeeding". 2013. Archived from the original on 8 September 2015. Retrieved 14 August 2015.
- ↑ Stevens, George M. Brenner, Craig W. (2013). Pharmacology (4th ed.). Philadelphia, PA: Elsevier/Saunders. p. 204. ISBN 978-1-4557-0278-7. Archived from the original on 2017-09-04.
- ↑ Engel, Jerome (2008). Epilepsy : a comprehensive textbook (2nd ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 1431. ISBN 978-0-7817-5777-5. Archived from the original on 2016-03-05.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- 1 2 Newton, CR (29 September 2012). "Epilepsy in poor regions of the world". Lancet. 380 (9848): 1193–201. doi:10.1016/S0140-6736(12)61381-6. PMID 23021288. S2CID 13933909. Archived from the original on 2 June 2020. Retrieved 12 March 2020.
- ↑ "Phenobarbital Generic Luminal". Archived from the original on 12 April 2021. Retrieved 1 October 2021.
- ↑ NICE (2005-10-27). "CG20 Epilepsy in adults and children: NICE guideline". NHS. Archived from the original on 2006-10-09. Retrieved 2006-09-06.
- 1 2 British National Formulary 51
- ↑ Nolan, Sarah J.; Tudur Smith, Catrin; Pulman, Jennifer; Marson, Anthony G. (2013-01-31). "Phenobarbitone versus phenytoin monotherapy for partial onset seizures and generalised onset tonic-clonic seizures". The Cochrane Database of Systematic Reviews (1): CD002217. doi:10.1002/14651858.CD002217.pub2. ISSN 1469-493X. PMID 23440786.
- ↑ Nevitt, Sarah J.; Marson, Anthony G.; Tudur Smith, Catrin (24 October 2018). "Carbamazepine versus phenobarbitone monotherapy for epilepsy: an individual participant data review". The Cochrane Database of Systematic Reviews. 10: CD001904. doi:10.1002/14651858.CD001904.pub4. ISSN 1469-493X. PMC 6517155. PMID 30353945.
- ↑ British Medical Association, Royal Pharmaceutical Society of Great Britain, Royal College of Paediatrics and Child Health and Neonatal and Paediatric Pharmacists Group (2006). "4.8.2 Drugs used in status epilepticus". British National Formulary for Children. London: BMJ Publishing. p. 269. ISBN 978-0-85369-676-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ Kälviäinen R, Eriksson K, Parviainen I (2005). "Refractory generalised convulsive status epilepticus : a guide to treatment". CNS Drugs. 19 (9): 759–68. doi:10.2165/00023210-200519090-00003. PMID 16142991. S2CID 11274515.
- ↑ Moshé, edited by Simon Shorvon, Emilio Perucca, Jerome Engel Jr.; foreword by Solomon (2009). The treatment of epilepsy (3rd ed.). Chichester, UK: Wiley-Blackwell. p. 587. ISBN 978-1-4443-1667-4. Archived from the original on 2016-05-21.
- ↑ British Medical Association, Royal Pharmaceutical Society of Great Britain, Royal College of Paediatrics and Child Health and Neonatal and Paediatric Pharmacists Group (2006). "4.8.1 Control of epilepsy". British National Formulary for Children. London: BMJ Publishing. pp. 255–6. ISBN 978-0-85369-676-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ John M. Pellock; W. Edwin Dodson; Blaise F. D. Bourgeois (2001-01-01). Pediatric Epilepsy. Demos Medical Publishing. p. 152. ISBN 978-1-888799-30-9.
- ↑ Raj D Sheth (2005-03-30). "Neonatal Seizures". eMedicine. WebMD. Archived from the original on 2006-07-09. Retrieved 2006-09-06.
- ↑ Booth D, Evans DJ (2004). Booth D (ed.). "Anticonvulsants for neonates with seizures". Cochrane Database of Systematic Reviews (3): CD004218. doi:10.1002/14651858.CD004218.pub2. PMID 15495087. Archived from the original on 2020-06-16. Retrieved 2006-09-06.
- ↑ Gold, Mark S.; Miller, Norman S. (July 1998). "Management of Withdrawal Syndromes and Relapse Prevention in Drug and Alcohol Abuse". American Family Physician. 58 (1): 139–46. PMID 9672434. Archived from the original on 2010-12-28. Retrieved 2011-03-31.
- ↑ Aschenbrenner, Diane S.; Venable, Samantha J. (2009). Drug Therapy in Nursing. Lippincott Williams & Wilkins. p. 277. ISBN 9780781765879.
- ↑ Kasper, Dennis (2015-04-08). Harrison's Principles of Internal Medicine, 19th edition. Mc Graw Hill. p. 2002. ISBN 978-0071802154.
Bilirubin concentrations during phenobarbital administration do not return to normal but are typically in the range of 51-86 µmol/L (3-5 mg/dL). Although the incidence of kernicterus in CN-II is low, instances have occurred, not only in infants but also in adolescents and adults, often in the setting of an intercurrent illness, fasting, or another factor that temporarily raises the serum bilirubin concentration above baseline and reduces serum albumin levels. For this reason, phenobarbital therapy is highly recommended, a single bedtime dose often sufficing to maintain clinically safe serum bilirubin concentrations.
- ↑ "Laboratory tests in Gilbert's syndrome after hepatitis". Archived from the original on 2021-08-28. Retrieved 2019-07-05.
- ↑ "Archived copy" (PDF). Archived (PDF) from the original on 2017-02-15. Retrieved 2017-06-07.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ López-Muñoz, F; Ucha-Udabe, R; Alamo, C (December 2005). "The history of barbiturates a century after their clinical introduction". Neuropsychiatric Disease and Treatment. 1 (4): 329–43. PMC 2424120. PMID 18568113.
Despite their widespread use during the first half of the 20th century, no barbiturate succeeded in eliminating the main drawbacks of these drugs, which were the phenomena of dependence and death by overdose
- ↑ "Barbiturate abuse in the United States, 1973". Archived from the original on 2018-05-08. Retrieved 2018-05-08.
- 1 2 3 4 "Phenobarbital - WikEM". www.wikem.org. Archived from the original on 24 September 2020. Retrieved 5 August 2020.
- 1 2 "Phenobarbital dosing, indications, interactions, adverse effects, and more". reference.medscape.com. Archived from the original on 2019-03-27. Retrieved 2019-03-27.
- ↑ Brodie, Martin J.; Mintzer, Scott; Pack, Alison M.; Gidal, Barry E.; Vecht, Charles J.; Schmidt, Dieter (January 2013). "Enzyme induction with antiepileptic drugs: Cause for concern?". Epilepsia. 54 (1): 11–27. doi:10.1111/j.1528-1167.2012.03671.x. PMID 23016553.
- 1 2 "PHENOBARBITAL injectable - Essential drugs". medicalguidelines.msf.org. Archived from the original on 26 November 2020. Retrieved 4 September 2020.
- ↑ Trimble MR; Cull C (1988). "Children of school age: the influence of antiepileptic drugs on behavior and intellect". Epilepsia. 29 Suppl 3: S15–9. doi:10.1111/j.1528-1157.1988.tb05805.x. PMID 3066616.
- 1 2 Rania Habal (2006-01-27). "Barbiturate Toxicity". eMedicine. WebMD. Archived from the original on 2008-07-20. Retrieved 2006-09-14.
- ↑ "Barbiturate intoxication and overdose". MedLine Plus. Archived from the original on 1 October 2008. Retrieved 15 July 2008.
- ↑ Lewis, Cassaundra B.; Adams, Ninos (2019), "Phenobarbital", StatPearls, StatPearls Publishing, PMID 30335310, archived from the original on 2021-08-28, retrieved 2019-03-27
- ↑ Brust, John C.M. (2014), "Alcohol and the nervous system – Chapter 8 – Acute withdrawal: diagnosis and treatment", Handbook of Clinical Neurology, Elsevier, 125: 123–131, doi:10.1016/b978-0-444-62619-6.00008-2, ISBN 9780444626196, PMID 25307572, archived from the original on 2019-03-28, retrieved 2019-03-28
- ↑ Flynn, Sean; Babi, M. Ali (2017), "Chapter 12: Anticonvulsants", Pharmacology and Therapeutics for Dentistry, Elsevier, pp. 176–192, doi:10.1016/b978-0-323-39307-2.00012-6, ISBN 9780323393072
- ↑ Sneader, Walter (2005-06-23). Drug Discovery. John Wiley and Sons. p. 369. ISBN 978-0-471-89979-2.
- ↑ Ole Daniel Enersen. "Alfred Hauptmann". Archived from the original on 2006-11-09. Retrieved 2006-09-06.
- ↑ Scott, Donald F (1993-02-15). The History of Epileptic Therapy. Taylor & Francis. pp. 59–65. ISBN 978-1-85070-391-4.
- ↑ Zoech, Irene (12 October 2003). "Named: the baby boy who was Nazis' first euthanasia victim". The Telegraph. Archived from the original on 16 December 2013. Retrieved 1 November 2013.
The case was to provide the rationale for a secret Nazi decree that led to 'mercy killings' of almost 300,000 mentally and physically handicapped people. The Kretschmars wanted their son dead but most of the other children were forcibly taken from their parents to be killed.
- ↑ Wesley J. Smith (26 March 2006). "Killing Babies, Compassionately". Weekly Standard. Archived from the original on 3 November 2013. Retrieved 1 November 2013.
Hitler later signed a secret decree permitting the euthanasia of disabled infants. Sympathetic physicians and nurses from around the country--many not even Nazi party members--cooperated in the horror that followed. Formal 'protective guidelines' were created, including the creation of a panel of 'expert referees,' which judged which infants were eligible for the program.
- ↑ Kaelber, Lutz (8 March 2013). "Kinderfachabteilung Ansbach". Sites of Nazi "Children's 'Euthanasia'" Crimes and Their Commemoration in Europe. University of Vermont. Archived from the original on 3 November 2013. Retrieved 1 November 2013.
In the late 1980s, important developments occurred at the clinic that led to the first publication on the subject and the display of two plaques. Dr Reiner Weisenseel wrote his dissertation under Dr Athen, then the director of the Ansbacher Bezirkskrankenhaus, on the involvement of the clinic in Euthanasia crimes, including the operation of the Kinderfachabteilung. In 1988 two members of the Green Party as well as the regional diet (Bezirkstag) were horrified to find portraits of physicians involved in Nazi euthanasia crimes among the honorary display of medical personnel in the administrative building, and they successfully petitioned to have these portraits removed. Since 1992 a plaque hangs in the entry hall way of the administrative building. It reads: 'In the Third Reich the Ansbach facility delivered to their death more than 2000 of the patients entrusted to it as life unworthy of living: They were transferred to killing facilities or starved to death. In their own way many people incurred responsibility.' It continues: 'Half a century later full of shame we commemorate the victims and call to remember the Fifth Commandment.' The killing of children specifically transferred to the clinic to be murdered is not noted. The plaque does not address that that euthanasia victims were not only starved or transported to gassing facilities but killed using barbiturates on site.
- ↑ Binder, Johann (October 2011). "Die Heil- und Pflegeanstalt Ansbach während des Nationalsozialismus" (PDF). Bezirksklinikum Ansbach (in German). Bezirkskliniken Mittelfranken. Archived from the original (PDF) on 3 November 2013. Retrieved 1 November 2013.
{{cite web}}: CS1 maint: unrecognized language (link) - ↑ Kaelber, Lutz (Spring 2013). "Jewish Children with Disabilities and Nazi "Euthanasia" Crimes" (PDF). The Bulletin of the Carolyn and Leonard Miller Center for Holocaust Studies. University of Vermont. Archived (PDF) from the original on 3 November 2013. Retrieved 1 November 2013.
Two Polish physicians reported at the time that 235 children from ages up to 14 were listed in the booklet, of whom 221 had died. An investigation revealed that the medical records of the children had been falsified, as those records showed a far lower dosage of Luminal given to them than was entered into the Luminal booklet. For example, the medical records for Marianna N. showed for 16 January 1943 (she died on that day) a dosage of 0.1 g of Luminal, whereas the Luminal booklet showed the actual dosage as 0.4 g, or four times the dosage recommended for her body weight.
- ↑ López-Muñoz, Francisco; Alamo, Cecilio; García-García, Pilar; Molina, Juan D.; Rubio, Gabriel (2008). "The role of psychopharmacology in the medical abuses of the Third Reich: From euthanasia programmes to human experimentation". Brain Research Bulletin. 77 (6): 388–403. doi:10.1016/j.brainresbull.2008.09.002. PMID 18848972. S2CID 39858807.
- ↑ Rachel Sheremeta Pepling (June 2005). "Phenobarbital". Chemical and Engineering News. 83 (25). Archived from the original on 2005-11-26. Retrieved 2006-09-06.
- ↑ John M. Pellock; W. Edwin Dodson; Blaise F. D. Bourgeois (2001-01-01). Pediatric Epilepsy. Demos Medical Publishing. p. 169. ISBN 978-1-888799-30-9.
- ↑ Robert Baumann (2005-02-14). "Febrile Seizures". eMedicine. WebMD. Archived from the original on 2006-09-06. Retrieved 2006-09-06.
- ↑ various (March 2005). "Diagnosis and management of epilepsies in children and young people" (PDF). Scottish Intercollegiate Guidelines Network. p. 15. Archived from the original (PDF) on 2006-06-10. Retrieved 2006-09-07.
- 1 2 Furniss, Brian; Hannaford, Antony; Smith, Peter; Tatchell, Austin (1996). Vogel's Textbook of Practical Organic Chemistry 5th Ed. London: Longman Science & Technical. pp. 1174–1179. ISBN 978-0-582-46236-6. Archived from the original on 2016-03-11.
- ↑ Adams, Roger (1957). Organic Reactions, Volume 9. New York: John Wiley & Sons, Inc. ISBN 978-0-471-00726-5. Archived from the original on 31 October 2014. Retrieved 18 July 2014.
- ↑ Adams, Roger; Thal, A. F. (1922). "Ethyl Phenylacetate". Organic Syntheses. 2: 27. doi:10.15227/orgsyn.002.0027.
- ↑ Meyer, G. M.; Levene, P. A. (1936). "Diethyl phenylmalonate". Organic Syntheses. 16: 33. doi:10.15227/orgsyn.016.0033.
- ↑ Chamberlain, J. S.; Chap, J.J.; Doyle, J. E.; Spaulding, L. B. (1935). "The Synthesis of 5,5-Alkylphenylbarbituric Acids". Journal of the American Chemical Society. 57 (2): 352–354. doi:10.1021/ja01305a036.
- ↑ Nelson, William L.; Cretcher, Leonard H. (1928). "The Preparation of Ethyl Phenylmalonate and of 5-Phenyl-beta-hydroxyethylbarbituric acid". Journal of the American Chemical Society. 50 (10): 2758–2762. doi:10.1021/ja01397a029.
- ↑ Makosza, M.; Jonczyk, A (1976). "Phase-Transfer Alkylation of Nitriles: 2-Phenylbutyronitrile". Organic Syntheses. 55: 91. doi:10.15227/orgsyn.055.0091.
- ↑ Marc, Inman Nyack; Haverstraw, N.; Bilter, William. "Preparation of Phenobarbital (US2358072, 1944)" (PDF). Google Patents. Kay-Fries Chemicals, Inc. Archived (PDF) from the original on 12 April 2016. Retrieved 30 October 2014.
- ↑ "DEA Diversion Control Division" (PDF). Archived (PDF) from the original on 2016-04-17. Retrieved 2016-04-13. pp 12 printed PDF 23.I.2016
- ↑ "DEA Diversion Control Division" (PDF). Archived (PDF) from the original on 2016-04-17. Retrieved 2016-04-13. page 1,7, 12, accessed 23.I.2016
- ↑ King, Wayne (April 19, 1989). "Abbie Hoffman Committed Suicide Using Barbiturates, Autopsy Shows". The New York Times. Archived from the original on October 16, 2007. Retrieved 2008-04-09.
- ↑ "Heaven's Gate cult members found dead". History Channel. Archived from the original on October 26, 2014. Retrieved September 17, 2014.
- ↑ Cheung, Helier (13 May 2017). "The mystery death haunting Norway for 46 years". BBC News. Archived from the original on 7 June 2019. Retrieved 18 June 2019.
- ↑ Thomas, WB (2003). "Seizures and narcolepsy". In Dewey, Curtis W. (ed.). A Practical Guide to Canine and Feline Neurology. Ames, Iowa: Iowa State Press. ISBN 978-0-8138-1249-6.
- ↑ Dodman, Nicholas. "Feline Hyperesthesia (FHS)". PetPlace.com. Archived from the original on 2011-11-23. Retrieved 2011-11-17.
- ↑ Editor, Cynthia M. Kahn; associate editor Scott Line (February 8, 2005). Kahn, Cynthia M., Line, Scott, Aiello, Susan E. (ed.) (ed.). The Merck Veterinary Manual (9th ed.). John Wiley & Sons. ISBN 978-0-911910-50-6.
{{cite book}}: CS1 maint: multiple names: authors list (link)
External links
| External sites: |
|
|---|---|
| Identifiers: |