Ethallobarbital
 | |
| Clinical data | |
|---|---|
| Other names | Aethallymal, Aethylal, Etallobarbital, Go 1067 |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Excretion | Renal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.017.412 |
| Chemical and physical data | |
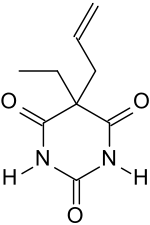
| Formula | C9H12N2O3 |
| Molar mass | 196.206 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Ethallobarbital (brand names Dormin, Dumex, Dormitiv, Dorval), also known as ethallymal and 5-allyl-5-ethylbarbituric acid, is an allyl-substituted barbiturate described as a sedative/hypnotic.[1][2][3][4][5] It was first synthesized in 1927.[1]
See also
References
- 1 2 Ganellin CR, Triggle DJ (21 November 1996). Dictionary of Pharmacological Agents. CRC Press. pp. 51–. ISBN 978-0-412-46630-4.
- ↑ Negwer M (1978). Organic-chemical drugs and their synonyms: an international survey. Akademie-Verlag. ISBN 978-0-89573-100-5.
- ↑ Muller NF, Dessing RP (19 June 1998). European Drug Index: European Drug Registrations (Fourth ed.). CRC Press. pp. 1440–. ISBN 978-3-7692-2114-5.
- ↑ Frigerio A, McCamish M (1980). Recent Developments in Mass Spectrometry in Biochemistry and Medicine. Elsevier Scientific Publishing Company. ISBN 9780444418708.
- ↑ Goldhahn H, Barth H (November 1953). "[Barbituric acids. II]". Pharmazie. 8 (11): 913–8. PMID 13133697.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.