Reposal
 | |
| Clinical data | |
|---|---|
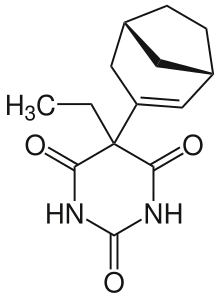
| Other names | Reposal, 5-Ethyl-5-(bicyclo(3.2.1)octenyl)barbituric acid |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H18N2O3 |
| Molar mass | 262.309 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Reposal is a barbiturate derivative invented in the 1960s in Denmark. It has sedative, hypnotic and anticonvulsant properties, and was used primarily for the treatment of insomnia.[1][2][3]
References
- ↑ Kessing SV, Tarding F, Thomsen AC (December 1963). "Reposal, A New Hypnotic. I. Pharmacodynamic Activity". Ugeskrift for Laeger (in Danish). 125: 1735–8. PMID 14103836.
- ↑ Kessing SV, Tarding F, Thomsen AC (December 1963). "Reposal, A New Hypnotic. Ii. Clinical Effects". Ugeskrift for Laeger (in Danish). 125: 1739–41. PMID 14103837.
- ↑ Nielsen P, Tarding F (1968). "The metabolic fate of 5-(bicyclo-3,2,1,-oct-2-en-2-yl)-5-ethyl barbituric acid, (Reposal)". Acta Pharmacologica et Toxicologica. 26 (6): 521–30. doi:10.1111/j.1600-0773.1968.tb00471.x. PMID 5756387.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.