Viqualine
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
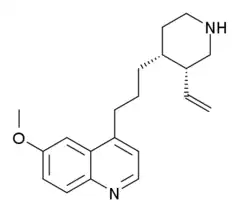
| Formula | C20H26N2O |
| Molar mass | 310.441 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Viqualine (INN) (developmental code name PK-5078) is an antidepressant and anxiolytic drug that was never marketed.[1][2][3] It acts as a potent and selective serotonin releasing agent and serotonin reuptake inhibitor.[3][4] In addition, viqualine displaces diazepam from the GABAA receptor and produces benzodiazepine-like effects, indicating that it is also a positive allosteric modulator of the benzodiazepine site of the GABAA receptor.[3][5] The drug has mainly been researched as a potential treatment for alcoholism.[6][7]
See also
References
- ↑ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 1–. ISBN 978-1-4757-2085-3.
- ↑ Faravelli C, Albanesi G, Sessarego A (1988). "Viqualine in resistant depression: a double-blind, placebo-controlled trial". Neuropsychobiology. 20 (2): 78–81. doi:10.1159/000118477. PMID 3075725.
- 1 2 3 George I. Papakostas; Maurizio Fava (2010). Pharmacotherapy for Depression and Treatment-resistant Depression. World Scientific. pp. 304–. ISBN 978-981-4287-59-3.
- ↑ Le Fur G, Imbault F, Mitrani N, Marquis F, Renault C, Dubroeucq MC, Gueremy C, Uzan A (February 1984). "The 5-hydroxytryptamine-releasing properties of two epimer quinoline derivatives". Neuropharmacology. 23 (2A): 169–73. doi:10.1016/S0028-3908(84)80010-6. PMID 6717757. S2CID 30380886.
- ↑ Faravelli, Carlo; Albanesi, Giorgio; Sessarego, Antonella (1988). "Viqualine in Resistant Depression: A Double-Blind, Placebo-Controlled Trial". Neuropsychobiology. 20 (2): 78–81. doi:10.1159/000118477. ISSN 1423-0224. PMID 3075725.
- ↑ Naranjo CA, Sellers EM (1989). "Serotonin uptake inhibitors attenuate ethanol intake in problem drinkers". Recent Developments in Alcoholism. 7: 255–66. doi:10.1007/978-1-4899-1678-5_13. ISBN 978-1-4899-1680-8. PMID 2522667.
- ↑ Naranjo CA, Sullivan JT, Kadlec KE, Woodley-Remus DV, Kennedy G, Sellers EM (September 1989). "Differential effects of viqualine on alcohol intake and other consummatory behaviors". Clinical Pharmacology and Therapeutics. 46 (3): 301–9. doi:10.1038/clpt.1989.142. PMID 2673621. S2CID 27080094.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.