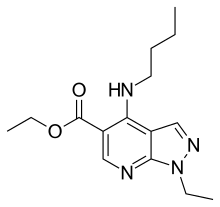
Cartazolate
 | |
| Clinical data | |
|---|---|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H22N4O2 |
| Molar mass | 290.367 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
Cartazolate (SQ-65,396) is a drug of the pyrazolopyridine class. It acts as a GABAA receptor positive allosteric modulator at the barbiturate binding site of the complex and has anxiolytic effects in animals.[1][2][3][4] It is also known to act as an adenosine antagonist at the A1 and A2 subtypes and as a phosphodiesterase inhibitor.[5][6] Cartazolate was tested in human clinical trials and was found to be efficacious for anxiety but was never marketed.[7] It was developed by a team at E.R. Squibb and Sons in the 1970s.[8]
Synthesis
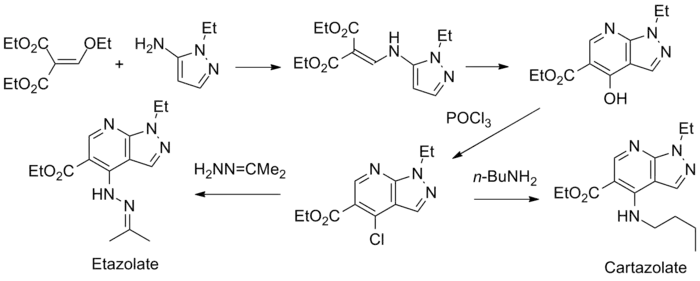
Condensation of aminopyrazole (1) with diethyl ethoxymethylenemalonate (2) gives the product of the addition-elimination (3). The product tautomerizes spontaneously to the hydroxypyridine (4). The hydroxyl group is then converted to the chloro-derivative by means of phosphorus oxychloride (5). Displacement of halogen by n-butylamine gives the antidepressant compound cartazolate. Displacement of halogen by the basic nitrogen of acetone hydrazone[10] affords the antidepressant etazolate.
See also
References
- ↑ Placheta P, Karobath M (March 1980). "In vitro modulation by SQ 20009 and SQ 65396 of GABA receptor binding in rat CNS membranes". European Journal of Pharmacology. 62 (2–3): 225–8. doi:10.1016/0014-2999(80)90281-2. PMID 6103810.
- ↑ Supavilai P, Karobath M (March 1981). "Action of pyrazolopyridines as modulators of [3H]flunitrazepam binding to the gaba/benzodiazepine receptor complex of the cerebellum". European Journal of Pharmacology. 70 (2): 183–93. doi:10.1016/0014-2999(81)90213-2. PMID 6114867.
- ↑ Leeb-Lundberg F, Snowman A, Olsen RW (May 1981). "Perturbation of benzodiazepine receptor binding by pyrazolopyridines involves picrotoxinin/barbiturate receptor sites". Journal of Neuroscience. 1 (5): 471–7. doi:10.1523/JNEUROSCI.01-05-00471.1981. PMC 6564167. PMID 7050308.
- ↑ Bristow DR, Martin IL (March 1990). "Biochemical characterization of an isolated and functionally reconstituted gamma-aminobutyric acid/benzodiazepine receptor". Journal of Neurochemistry. 54 (3): 751–61. doi:10.1111/j.1471-4159.1990.tb02315.x. PMID 2154549. S2CID 25784613.
- ↑ Daly JW, Hong O, Padgett WL, Shamim MT, Jacobson KA, Ukena D (February 1988). "Non-xanthine heterocycles: activity as antagonists of A1- and A2-adenosine receptors". Biochemical Pharmacology. 37 (4): 655–64. doi:10.1016/0006-2952(88)90139-6. PMC 3445624. PMID 2829919.
- ↑ Wachtel H (1982). "Characteristic behavioural alterations in rats induced by rolipram and other selective adenosine cyclic 3', 5'-monophosphate phosphodiesterase inhibitors". Psychopharmacology. 77 (4): 309–16. doi:10.1007/BF00432761. PMID 6182575. S2CID 10122417.
- ↑ O'Brien, Robert (1986). Receptor binding in drug research. New York: Dekker. p. 519. ISBN 0-8247-7548-1.
- ↑ US 3966746, Hoehn, Hans & Denzel, Theodor, "Amino derivatives of pyrazolopyridine carboxamides", published 1976-06-29, assigned to Squibb & Sons Inc.
- ↑ DE 2123318, Hoehn, Hans & Denzel, Theodor, "Aminoderivate von Pyrazolopyridincarbonsäuren, deren Estern und Salzen, Verfahren zu ihrer Herstellung und ihre Verwendung [Amino derivatives of pyrazolopyridinecarboxylic acids, their esters and salts, processes for their preparation and their use]", published 1971-12-09, assigned to E.R. Squibb & Sons Inc.
- ↑ "Organic Syntheses Procedure".