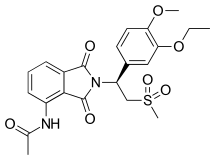
Apremilast
 | |
 | |
| Names | |
|---|---|
| Pronunciation | /əˈprɛmɪlæst/ ə-PREM-i-last |
| Trade names | Otezla, Aplex, others |
| Other names | CC-10004 |
IUPAC name
| |
| Clinical data | |
| Drug class | Phosphodiesterase 4 (PDE4) inhibitor[1] |
| Main uses | Plaque psoriasis, psoriatic arthritis, Behçet's disease[1][2] |
| Side effects | Diarrhea, nausea, common cold, headaches[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth (tablets) |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a614022 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 73%;[5] Tmax = ~2.5 hours |
| Protein binding | c. 68%[5] |
| Metabolism | Liver (CYP3A4, with minor contributions from CYP2A6, CYP1A2)[5] |
| Metabolites | O-desmethylapremilast glucuronide (and others)[6] |
| Elimination half-life | 6–9 hours[5] |
| Excretion | Urine (58%), faeces (39%)[5] |
| Chemical and physical data | |
| Formula | C22H24N2O7S |
| Molar mass | 460.50 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Apremilast, sold under the brand name Otezla among others, is a medication used to treat plaque psoriasis, psoriatic arthritis, and Behçet's disease.[1][2] It is used when other treatments are not effective.[1] It is taken by mouth.[7]
Common side effects include diarrhea, nausea, the common cold, and headaches.[1] Other side effects may include depression and weight loss.[2] Safety in pregnancy is unclear, with evidence of harm in other animals.[8] It is an inhibitor of the enzyme phosphodiesterase 4 (PDE4) which results in a decrease in cytokins.[1]
Apremilast was approved for medical use in the United States in 2014 and Europe in 2015.[2][1] In the United Kingdom 4 weeks of treatment costs the NHS about £550 as of 2021.[7] In the United States this amount costs about 4,100 USD.[9]
Medical uses

Apremilast is indicated in the United States for the treatment of adults with active psoriatic arthritis, people with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, and adults with oral ulcers associated with Behçet's disease.[10]
In the European Union, apremilast alone or in combination with disease-modifying antirheumatic drugs (DMARDs), is indicated for the treatment of active psoriatic arthritis (PsA) in adults who have had an inadequate response or who have been intolerant to a prior DMARD therapy.[11] It is also indicated for the treatment of moderate to severe chronic plaque psoriasis in adults who failed to respond to, have a contraindication to, or are intolerant of other systemic therapies, including cyclosporine, methotrexate, or psoralen and ultraviolet-A light.[11]
Dosage
It is started at a dose of 10 mg and than increased to 30 mg twice per day over a week.[1] Lower doses are used in those with kidney problems.[1] If it is not effective after 6 months it should be stopped.[1]
Contraindications
In the European Union, the drug is contraindicated during pregnancy because mice and monkeys receiving very high doses of apremilast have been observed to suffer miscarriages and other pregnancy problems.[6] In the U.S., it may be used for pregnant women "if the potential benefit justifies the potential risk to the fetus".[3]
Side effects
Diarrhoea and vomiting
Diarrhoea occurs in about 25% of people taking apremilast. Severe gastrointestinal symptoms, when they occur, typically start within the first few weeks of treatment.[12][13]
Psychological
Worsening depression, suicidal thoughts, and other mood changes may occur with apremilast.[10]
Weight loss
Weight loss has been associated with apremilast. Reports from clinical studies indicated a 5 to 10% decrease in body weight in 10% of patients taking apremilast (compared to 3.3% of patients taking placebo).[10]
Other
Common, usually mild to moderate adverse effects associated with apremilast include headache, back pain, nausea, diarrhoea, fatigue, nasopharyngitis, and upper respiratory tract infections.[14]
Interactions
Concurrent use of strong cytochrome P450 enzyme inducers has been shown to decrease exposure of apremilast and can result in reduced or loss of efficacy of apremilast. Using it simultaneously with strong P450 enzyme inducers, including rifampicin, phenobarbital, carbamazepine, phenytoin,[10] and St. John's wort is not recommended.[15]
Pharmacology
Mechanism of action
Apremilast is a small-molecule inhibitor of PDE4,[10] an enzyme that breaks down cyclic adenosine monophosphate (cAMP).[10] In inflammatory cells, PDE4 is the dominant enzyme responsible for this reaction. The resulting increase in cAMP levels down-regulates expression of a number of pro-inflammatory factors such as tumor necrosis factor alpha (TNFα), interleukin 17, interleukin 23, and many others, and up-regulates the anti-inflammatory interleukin 10. In ex vivo models of arthritis, IL-12/IL-23p40 was specifically identified as a downstream target of apremilast.[16] The importance of these individual factors for the clinical effect of apremilast is not clear.[6]
Pharmacokinetics
Apremilast is absorbed well from the gut (73%), independently of food intake, and reaches peak blood plasma concentrations after 2.5 hours. Plasma protein binding is 68%. It is metabolised in the liver, mainly via the enzyme CYP3A4, but to a minor extent via CYP1A2 and CYP2A6. The main metabolite is O-desmethylapremilast glucuronide.[5][6]
Its half-life is 6–9 hours. The substance is eliminated through the kidney (58%) and feces (39%), mainly in form of its metabolites. Only 3% of the original substance is found in the urine, and 7% in the feces.[5][6]
Chemistry
Apremilast is a phthalimide derivative. It is a white to pale yellow, nonhygroscopic powder that is practically insoluble in water and buffer solutions in a wide pH range, but is soluble in lipophilic solvents such as acetone, acetonitrile, butanone, dichloromethane, and tetrahydrofuran.[17]
In vitro, apremilast reduces PDE4 activity, leading to an increase in cyclic-adenosine monophosphate (cAMP) concentrations in immune and nonimmune cell types, partially inhibiting the production of many pro-inflammatory cytokines, such as TNF-α, IFN-γ IL-2, IL-12, and IL-23 and elevating the production of the anti-inflammatory cytokine IL-10.[18][19] The inhibition potency of apremilast in TNF-α production is similar to lenalidomide.[20]
Celgene reported seven kinds of crystal forms - A, B, C, D, E, F, and G -nand thought the crystal form B was the most thermodynamically stable anhydrous form. However, Utopharm reported another more thermodynamically stable anhydrous crystal form II than the crystal form B.[21]
History
Apremilast was approved by the US Food and Drug Administration (FDA) in 2014, for treatment of adults with active psoriatic arthritis and moderate to severe plaque psoriasis, and approved in 2019, for oral ulcers associated with Behçet's disease.[22][23][24] Apremilast is taken by mouth.[25]
Apremilast was approved for use in the European Union in January 2015.[11]
In 2019, Amgen acquired Otezla from Celgene for US$13.4 billion.[26]
In 2020, Otezla generated US$2.2 billion for Amgen.[27]
Society and culture
Otezla is available in the U.S., but is dispensed only through a network of specialty pharmacies.[22] The estimated wholesale price is $22,500 for a year of treatment.[25] In Austria, the drug is available in all pharmacies, and a year of treatment costs health insurances about €11,000.[28] In India, the drug is available in all pharmacies, and a year of treatment costs about ₹22,000. Celgene made Otezla available in the U.K. in May 2021.[29]
See also
References
- 1 2 3 4 5 6 7 8 9 10 11 "Otezla". Archived from the original on 3 March 2020. Retrieved 15 January 2022.
- 1 2 3 4 "DailyMed - OTEZLA- apremilast tablet, film coated". dailymed.nlm.nih.gov. Archived from the original on 15 January 2022. Retrieved 15 January 2022.
- 1 2 "Apremilast (Otezla) Use During Pregnancy". Drugs.com. 27 September 2019. Archived from the original on 4 December 2020. Retrieved 19 April 2020.
- ↑ "Otezla 30 mg Film-Coated Tablets - Summary of Product Characteristics (SmPC)". (emc). 15 April 2020. Archived from the original on 15 January 2021. Retrieved 19 April 2020.
- 1 2 3 4 5 6 7 "Otezla (aprelimast) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 17 October 2014. Retrieved 28 March 2014.
- 1 2 3 4 5 Haberfeld H, ed. (2015). Austria-Codex (in Deutsch). Vienna: Österreichischer Apothekerverlag.
- 1 2 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1165. ISBN 978-0857114105.
- ↑ "Apremilast (Otezla) Use During Pregnancy". Drugs.com. Archived from the original on 4 December 2020. Retrieved 15 January 2022.
- ↑ "Otezla Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 25 May 2016. Retrieved 15 January 2022.
- 1 2 3 4 5 6 "Otezla- apremilast tablet, film coated Otezla- apremilast kit". DailyMed. 26 February 2020. Archived from the original on 21 January 2021. Retrieved 19 April 2020.
- 1 2 3 "Otezla EPAR". European Medicines Agency (EMA). Archived from the original on 3 March 2020. Retrieved 19 April 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ Kavanaugh A, Mease PJ, Gomez-Reino JJ, Adebajo AO, Wollenhaupt J, Gladman DD, et al. (March 2015). "Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis". The Journal of Rheumatology. 42 (3): 479–88. doi:10.3899/jrheum.140647. PMID 25593233.
- ↑ Stein Gold L, Bagel J, Lebwohl M, Jackson JM, Chen R, Goncalves J, et al. (February 2018). "Efficacy and Safety of Apremilast in Systemic- and Biologic-Naive Patients With Moderate Plaque Psoriasis: 52-Week Results of UNVEIL". Journal of Drugs in Dermatology. 17 (2): 221–228. PMID 29462231.
- ↑ Mease PJ, Armstrong AW (March 2014). "Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis". Drugs. 74 (4): 423–41. doi:10.1007/s40265-014-0191-y. PMC 3958815. PMID 24566842.
- ↑ "Otezla Product Monograph" (PDF). Celgene Canada. Celgene Corporation. Archived from the original (PDF) on 7 April 2015. Retrieved 3 April 2015.
- ↑ Kragstrup TW, Adams M, Lomholt S, Nielsen MA, Heftdal LD, Schafer P, Deleuran B (2019). "ex vivo models of arthritis". Therapeutic Advances in Musculoskeletal Disease. 11: 1759720X19828669. doi:10.1177/1759720X19828669. PMC 6391542. PMID 30833991.
- ↑ "Assessment report for Otezla" (PDF). EMA. 20 November 2014. Archived (PDF) from the original on 17 June 2018. Retrieved 8 September 2021.
- ↑ Schafer P (June 2012). "Apremilast mechanism of action and application to psoriasis and psoriatic arthritis". Biochemical Pharmacology. 83 (12): 1583–90. doi:10.1016/j.bcp.2012.01.001. PMID 22257911.
- ↑ Schafer PH, Parton A, Gandhi AK, Capone L, Adams M, Wu L, et al. (February 2010). "Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis". British Journal of Pharmacology. 159 (4): 842–55. doi:10.1111/j.1476-5381.2009.00559.x. PMC 2829210. PMID 20050849.
- ↑ Michelli ML (2011). Liver cirrhosis : causes, diagnosis, and treatment. New York: Nova Biomedical Books. ISBN 978-1-61209-248-5. Archived from the original on 4 March 2016. Retrieved 8 September 2021.
- ↑ "A novel stable and non-solvate crystal form II on Apremilast and processes for the preparation thereof". Utopharm. 18 April 2015. Archived from the original on 31 May 2015.
- 1 2 "Oral Otezla (apremilast) Approved by the U.S. Food and Drug Administration for the Treatment of Patients with Moderate to Severe Plaque Psoriasis" Archived 6 April 2015 at the Wayback Machine (Press release). Celgene Corporation. 23 September 2014. Retrieved 29 October 2014.
- ↑ "FDA approves Otezla to treat psoriatic arthritis". U.S. Food and Drug Administration (FDA) (Press release). 21 March 2014. Archived from the original on 13 February 2017. Retrieved 19 April 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "FDA Approves OTEZLA (apremilast) for the Treatment of Oral Ulcers Associated with Behçet's Disease". Celgene Corporation (Press release). 19 July 2019. Archived from the original on 20 April 2020. Retrieved 11 November 2019.
- 1 2 Apremilast for the Treatment of Psoriatic Arthritis American College of Rheumatology (14 June 2014). Retrieved 29 October 2014. Archived 21 February 2015 at the Wayback Machine
- ↑ "Amgen To Acquire Otezla For $13.4 Billion In Cash or Approximately $11.2 Billion Net of Anticipated Future Cash Tax Benefits". Amgen, Inc. 26 August 2019. Archived from the original on 3 October 2021. Retrieved 19 April 2020.
- ↑ "Amgen Reports Fourth Quarter And Full Year 2020 Financial Results". Amgen, Inc. 2 February 2021. Archived from the original on 3 February 2021. Retrieved 2 February 2021.
- ↑ "Warenverzeichnis" (in Deutsch). I. Österreichischer Apothekerverlag. January 2016.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ "Celgene launches new psoriasis drug Otezla in UK". 24 May 2021. Archived from the original on 24 May 2021. Retrieved 24 May 2021.
External links
| External sites: |
|
|---|---|
| Identifiers: |