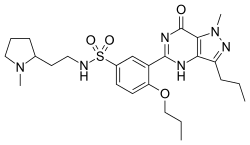
Udenafil
 | |
| Clinical data | |
|---|---|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 93,9% |
| Metabolism | Liver (mainly CYP3A4) |
| Elimination half-life | 7.3–12.1 hours |
| Excretion | Biliary |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C25H36N6O4S |
| Molar mass | 516.66 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
The drug udenafil is marketed under the trade name Zydena. It is within the PDE5 inhibitor class (which also includes avanafil, sildenafil, tadalafil, and vardenafil). Like other PDE5 inhibitors, it is used to treat erectile dysfunction. Udenafil was developed by Dong-A Pharmaceutical.[1] It has fairly rapid onset of action (peak plasma concentration after 1 to 1.5 hours), and has long duration of action (plasma half-life of 11 to 13 hours). Udenafil's pharmacokinetics allows once-daily dosage (in addition to on-demand use).[2] Typical doses are 100 and 200 mg. Udenafil is available in Korea, Russia, and the Philippines.[3] It has not yet been approved for use in the United States by the U.S. Food and Drug Administration.
References
- ↑ Zydena (udenafil) product-information page. Dong-A Pharmaceutical. Retrieved on April 13, 2009.
- ↑ Kang SG, Kim JJ (April 2013). "Udenafil: efficacy and tolerability in the management of erectile dysfunction". Therapeutic Advances in Urology. 5 (2): 101–10. doi:10.1177/1756287212470019. PMC 3607490. PMID 23554845.
- ↑ "Zydena". Drugs.com.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.