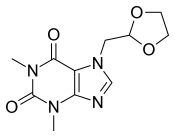
Doxofylline
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.067.468 |
| Chemical and physical data | |
| Formula | C11H14N4O4 |
| Molar mass | 266.257 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Doxofylline (also known as doxophylline) is a xanthine derivative drug used in the treatment of asthma.[1]
Medical uses
It has antitussive and bronchodilator[2] effects, and acts as a phosphodiesterase inhibitor.[3] Doxophylline is used to treat asthma, COPD and bronchospasm.[4]
In animal and human studies, it has shown similar efficacy to theophylline but with significantly fewer side effects.[5] In February 2014, the US FDA granted an orphan drug designation to doxofylline for the treatment of bronchiectasis following the submission of an application by Alitair Pharmaceuticals, in May 2013.[6][7][8]
Chemistry
Unlike other xanthines, doxofylline lacks any significant affinity for adenosine receptors and does not produce stimulant effects. This suggests that its antiasthmatic effects are mediated by another mechanism, perhaps its actions on phosphodiesterase.[1] From a pharmacokinetic point of view, doxofylline importantly differs from theophylline also because it lacks the ability to interfere with the cytochrome enzymes CYP1A2, CYP2E1 and CYP3A4, thus preventing significant interaction with other drugs metabolized via these pathways in the liver. [9][10]
Names
It is marketed under many brand names worldwide, including: Xiva, An Li Nuo Er, An Sai Ma, Ansimar, Asima, Bestofyline, Chuan Ning, D-Fyal, Dilatair, Doxiba, Doxobid, Doxobron, Doxofilina, Doxofillina, Doxofyllin, Doxoll, Doxophylline, Doxovent, Doxyjohn, Fei Te Ai Si, Jian Fang Neng, Lang Ming, Lv Meng, Mai Ping Xi, Maxivent, Mucosma, Na De Lai, Phylex, Phyllin, Puroxan, Rexipin, Shu Zhi, Shuai An, Shuweixin, Suo Di, Suo Ji, Suo Li An, Xi Si Nuo, Xin Qian Ping, Xin Xi Ping, Yi Suo, and Yili.[11]
It is also marketed as a combination drug with terbutaline as Doxoll-TL, Mucosma-T and Phylex-TR.[11]
It is also marketed as a combination drug with montelukast as Doxoll-ML, Doxomont, Doxoril-M, Doxovent-M, Lunair-M, and Venidox-M.[11]
References
- 1 2 Cirillo R, Barone D, Franzone JS (1988). "Doxofylline, an antiasthmatic drug lacking affinity for adenosine receptors". Archives Internationales de Pharmacodynamie et de Therapie. 295: 221–37. PMID 3245738.
- ↑ Poggi R, Brandolese R, Bernasconi M, Manzin E, Rossi A (October 1989). "Doxofylline and respiratory mechanics. Short-term effects in mechanically ventilated patients with airflow obstruction and respiratory failure". Chest. 96 (4): 772–8. doi:10.1378/chest.96.4.772. PMID 2791671. Archived from the original on 2013-04-14.
- ↑ Dini FL, Cogo R (2001). "Doxofylline: a new generation xanthine bronchodilator devoid of major cardiovascular adverse effects". Current Medical Research and Opinion. 16 (4): 258–68. doi:10.1185/030079901750120196. PMID 11268710.
- ↑ Cazzola M, Calzetta L, Rogliani P, Page C, Matera MG (August 2018). "Impact of doxofylline in COPD: A pairwise meta-analysis" (PDF). Pulmonary Pharmacology & Therapeutics. 51: 1–9. doi:10.1016/j.pupt.2018.04.010. PMID 29705620.
- ↑ Sankar J, Lodha R, Kabra SK (March 2008). "Doxofylline: The next generation methylxanthine". Indian Journal of Pediatrics. 75 (3): 251–4. doi:10.1007/s12098-008-0054-1. PMID 18376093.
- ↑ "Orphan Drug Designations and Approvals List as of 12‐01‐2014" (PDF).
- ↑ SMI Support. "News Release 10/26/15". www.alitair.com. Retrieved 2018-09-16.
- ↑ "Doxofylline - AdisInsight". adisinsight.springer.com. Retrieved 2018-09-16.
- ↑ Mennini FS, Sciattella P, Marcellusi A, Marcobelli A, Russo A, Caputi AP (October 2017). "Treatment plan comparison in acute and chronic respiratory tract diseases: an observational study of doxophylline vs. theophylline" (PDF). Expert Review of Pharmacoeconomics & Outcomes Research. 17 (5): 503–510. doi:10.1080/14737167.2017.1301815. hdl:2108/194830. PMID 28277853.
- ↑ Matera MG, Page C, Cazzola M (2017-12-05). "Doxofylline is not just another theophylline!". International Journal of Chronic Obstructive Pulmonary Disease. 12: 3487–3493. doi:10.2147/COPD.S150887. PMC 5723117. PMID 29255355.
- 1 2 3 "Doxofylline - international brand names". Drugs.com. Retrieved 18 January 2017.
Further reading
- Shukla D, Chakraborty S, Singh S, Mishra B (October 2009). "Doxofylline: a promising methylxanthine derivative for the treatment of asthma and chronic obstructive pulmonary disease". Expert Opinion on Pharmacotherapy. 10 (14): 2343–56. doi:10.1517/14656560903200667. PMID 19678793.