Sulfoaildenafil
 | |
| Names | |
|---|---|
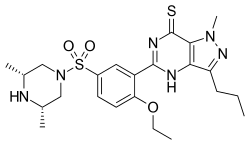
| IUPAC name
5-(5-(((3R,5S)-3,5-Dimethylpiperazin-1-yl)sulfonyl)-2-ethoxyphenyl)-1-methyl-3-propyl-1H-pyrazolo[4,3-d]pyrimidine-7(4H)-thione | |
| Other names
Thioaildenafil; Thiomethisosildenafil | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C23H32N6O3S2 |
| Molar mass | 504.67 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sulfoaildenafil (thioaildenafil) is a synthetic chemical compound that is a structural analog of sildenafil (Viagra).[1] It was first reported in 2005,[2] and it is not approved by any health regulation agency. Like sildenafil, sulfoaildenafil is a phosphodiesterase type 5 inhibitor.
Sulfoaildenafil has been found as an adulterant in a variety of supplements which are sold as "natural" or "herbal" sexual enhancement products.[3][4][5][6] A range of designer analogues of USA FDA-approved inhibitors of type-5 cGMP-specific phosphodiesterase (PDE5), such as sildenafil and vardenafil, have been detected in recent years as adulterants in over-the-counter herbal aphrodisiac products and dietary supplements,[7][8][9] in an apparent attempt to circumvent both the legal restrictions on sale of erectile dysfunction drugs, which are prescription-only medicines in most Western countries, and the patent protection which prevents sale of these drugs by competitors except under license to their inventors. These compounds have been demonstrated to display PDE5 inhibitory activity in vitro and presumably have similar effects when consumed, but have undergone no formal testing in either humans or animals, and as such represent a significant health risk to consumers of these products due to their unknown safety profile.[10] Some attempts have been made to ban these drugs as unlicensed medicines, but progress has been slow so far, as even in those jurisdictions which have laws targeting designer drugs, the laws are drafted to ban analogues of illegal drugs of abuse, rather than analogues of prescription medicines. However at least one court case has resulted in a product being taken off the market.[11]
In December 2010, the United States Food and Drug Administration (FDA) issued a warning to consumers about such products stating, "The FDA has found many products marketed as dietary supplements for sexual enhancement during the past several years that can be harmful because they contain active ingredients in FDA-approved drugs or variations of these ingredients."[12]
See also
References
- ↑ Gratz, SR; Zeller, M; Mincey, DW; Flurer, CL (2009). "Structural characterization of sulfoaildenafil, an analog of sildenafil". Journal of Pharmaceutical and Biomedical Analysis. 50 (2): 228–31. doi:10.1016/j.jpba.2009.04.003. PMID 19427155.
- ↑ WO 2005058899, Li, Shuxin; Ren, Jianping & Zhao, Yanjin et al., "Pyrazolopyrimidinethione Derivatives, Salts and Solvates thereof, Preparation Methods and Use thereof", published 2005-06-30, assigned to Academy of Military Medical Sciences
- ↑ Gryniewicz, CM; Reepmeyer, JC; Kauffman, JF; Buhse, LF (2009). "Detection of undeclared erectile dysfunction drugs and analogues in dietary supplements by ion mobility spectrometry". Journal of Pharmaceutical and Biomedical Analysis. 49 (3): 601–6. doi:10.1016/j.jpba.2008.12.002. PMID 19150190.
- ↑ FDA warns consumers to avoid sexual enhancement pills, Sanjay Gupta, CNN, December 13th, 2010
- ↑ Reepmeyer JC, d'Avignon DA (January 2009). "Structure elucidation of thioketone analogues of sildenafil detected as adulterants in herbal aphrodisiacs". Journal of Pharmaceutical and Biomedical Analysis. 49 (1): 145–50. doi:10.1016/j.jpba.2008.10.007. PMID 19042103.
- ↑ Balayssac S, Trefi S, Gilard V, Malet-Martino M, Martino R, Delsuc MA (November 2008). "2D and 3D DOSY (1)H NMR, a useful tool for analysis of complex mixtures: Application to herbal drugs or dietary supplements for erectile dysfunction". Journal of Pharmaceutical and Biomedical Analysis. 50 (4): 602–12. doi:10.1016/j.jpba.2008.10.034. PMID 19108978.
- ↑ Zou P, Oh SS, Hou P, Low MY, Koh HL (February 2006). "Simultaneous determination of synthetic phosphodiesterase-5 inhibitors found in a dietary supplement and pre-mixed bulk powders for dietary supplements using high-performance liquid chromatography with diode array detection and liquid chromatography-electrospray ionization tandem mass spectrometry". J Chromatogr A. 1104 (1–2): 113–22. doi:10.1016/j.chroma.2005.11.103. PMID 16364350.
- ↑ Gratz SR, Gamble BM, Flurer RA (2006). "Accurate mass measurement using Fourier transform ion cyclotron resonance mass spectrometry for structure elucidation of designer drug analogs of tadalafil, vardenafil and sildenafil in herbal and pharmaceutical matrices". Rapid Commun. Mass Spectrom. 20 (15): 2317–27. Bibcode:2006RCMS...20.2317G. doi:10.1002/rcm.2594. PMID 16817245.
- ↑ Hou P, Zou P, Low MY, Chan E, Koh HL (September 2006). "Structural identification of a new acetildenafil analogue from pre-mixed bulk powder intended as a dietary supplement". Food Addit Contam. 23 (9): 870–5. doi:10.1080/02652030600803856. PMID 16901855. S2CID 35240702.
- ↑ Oh, SS; Zou, P; Low, MY; Koh, HL (2006). "Detection of sildenafil analogues in herbal products for erectile dysfunction". Journal of Toxicology and Environmental Health. Part A. 69 (21): 1951–8. doi:10.1080/15287390600751355. PMID 16982533. S2CID 40831895.
- ↑ Venhuis, BJ; Blok-Tip, L; De Kaste, D (2008). "Designer drugs in herbal aphrodisiacs". Forensic Science International. 177 (2–3): e25–7. doi:10.1016/j.forsciint.2007.11.007. PMID 18178354.
- ↑ FDA warns consumers to avoid Man Up Now capsules, United States Food and Drug Administration, Dec. 15, 2010