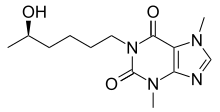
Lisofylline
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 1-(5-Hydroxyhexyl)-3,7-dimethylxanthine (HDX) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C13H20N4O3 |
| Molar mass | 280.328 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Lisofylline (LSF) is a synthetic small molecule with novel anti-inflammatory properties. LSF can effectively prevent type 1 diabetes in preclinical models and improves the function and viability of isolated or transplanted pancreatic islets. It is a metabolite of pentoxifylline.
As well, LSF improves cellular mitochondrial function and blocks interleukin-12 (IL-12) signaling and STAT-4 activation in target cells and tissues. IL-12 and STAT-4 activation are important pathways linked to inflammation and autoimmune damage to insulin producing cells. Therefore, LSF and related analogs could provide a new therapeutic approach to prevent or reverse type 1 diabetes. LSF also directly reduces glucose-induced changes in human kidney cells suggesting that LSF and analogs have the potential to treat the complications associated with diabetes.
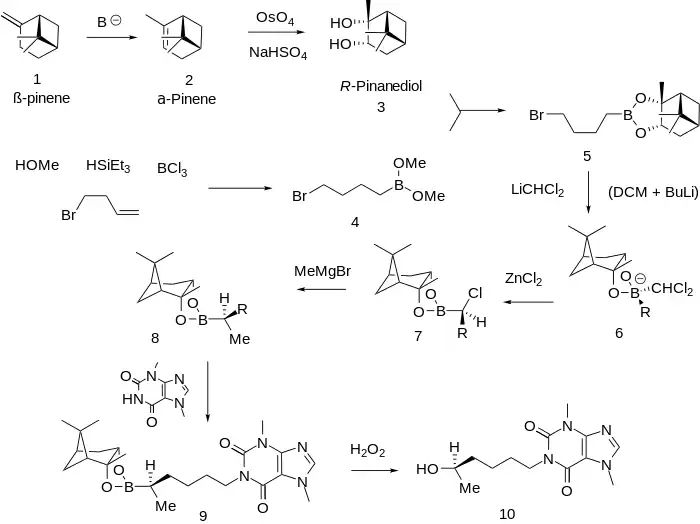
Synthesis
The R enantiomer of the pentoxyfylline analogue in which the ketone has been reduced to an alcohol shows enhanced activity as an inhibitor of acetyl CoA over the parent drug.
For analogs see:[8]
Further reading
- DE 3942872, Aretz, Werner; Furrer, Harald & Gebert, Ulrich et al., "Verfahren zur enantioselektiven Darstellung von (ω-1)-Hydroxyalkylxanthinen [Method for the enantioselective preparation of(ω-1)-hydroxyalkylxanthines]", published 1991-06-27, assigned to Hoechst AG
- US 5310666, Aretz, Werner; Furrer, Harald & Gebert, Ulrich et al., "Process for the enantioselective preparation of (β-1)-hydroxyalkylxanthines by reduction using Rhodotorula rubra", published 1994-05-10, assigned to Hoechst AG
- WO 9531450, Klein, J. Peter; Leigh, Alistair J. & Michnick, John et al., "Asymmetric synthesis of chiral secondary alcohols", published 1995-11-23, assigned to Cell Therapeutics Inc.
References
- ↑ Matteson DS, Sadhu KM, Peterson ML (1986). "99% Chirally selective synthesis via pinanediol boronic esters: Insect pheromones, diols, and an amino alcohol". Journal of the American Chemical Society. 108 (4): 810. doi:10.1021/ja00264a039.
- ↑ Matteson DS, Ray R, Rocks RR, Tsai DJ (1980). "Directed chiral synthesis with pinanediol boronic esters". Journal of the American Chemical Society. 102 (25): 7590. doi:10.1021/ja00545a046.
- ↑ Matteson DS, Jesthi PK, Sadhu KM (1997). "Asymmetric synthesis of alkylarylcarbinols via reaction of a chiral pinanediol alkylboronic ester with arylmethyl chlorides". Tetrahedron: Asymmetry. 8 (23): 3843. doi:10.1016/S0957-4166(97)00565-X.
- ↑ Matteson DS, Jesthi PK, Sadhu KM (1984). "Synthesis and properties of pinanediol .alpha.-amido boronic esters". Organometallics. 3 (8): 1284. doi:10.1021/om00086a024.
- ↑ Matteson DS (1988). "Asymmetric synthesis with boronic esters". Accounts of Chemical Research. 21 (8): 294–300. doi:10.1021/ar00152a002.
- ↑ Matteson DS (October 2013). "Boronic esters in asymmetric synthesis". The Journal of Organic Chemistry. 78 (20): 10009–23. doi:10.1021/jo4013942. PMID 23875690.
- ↑ Scott HK, Aggarwal VK (November 2011). "Highly enantioselective synthesis of tertiary boronic esters and their stereospecific conversion to other functional groups and quaternary stereocentres". Chemistry. 17 (47): 13124–32. doi:10.1002/chem.201102581. PMID 22052475.
- ↑ Cui P, Macdonald TL, Chen M, Nadler JL (July 2006). "Synthesis and biological evaluation of lisofylline (LSF) analogs as a potential treatment for Type 1 diabetes". Bioorganic & Medicinal Chemistry Letters. 16 (13): 3401–5. doi:10.1016/j.bmcl.2006.04.036. PMID 16650991.