Trapidil
 | |
| Clinical data | |
|---|---|
| Trade names | Rocornal, Avantrin, Travisco |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.035.834 |
| Chemical and physical data | |
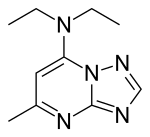
| Formula | C10H15N5 |
| Molar mass | 205.265 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Trapidil is a vasodilator and an antiplatelet drug.[1][2] It also acts as an antagonist of platelet-derived growth factor.[3]
An analog was assigned the codename AR 12-456.
References
- ↑ Büyükafşar K, Yazar A, Düşmez D, Oztürk H, Polat G, Levent A (October 2001). "Effect of trapidil, an antiplatelet and vasodilator agent on gentamicin-induced nephrotoxicity in rats". Pharmacological Research. 44 (4): 321–8. doi:10.1006/phrs.2001.0864. PMID 11592868.
- ↑ Liu M, Sun Q, Wang Q, Wang X, Lin P, Yang M, Yan Y (March–April 2014). "Effect of trapidil in myocardial ischemia-reperfusion injury in rabbit". Indian Journal of Pharmacology. 46 (2): 207–10. doi:10.4103/0253-7613.129320. PMC 3987192. PMID 24741195.
- ↑ Maresta A, Balducelli M, Cantini L, Casari A, Chioin R, Fabbri M, et al. (December 1994). "Trapidil (triazolopyrimidine), a platelet-derived growth factor antagonist, reduces restenosis after percutaneous transluminal coronary angioplasty. Results of the randomized, double-blind STARC study. Studio Trapidil versus Aspirin nella Restenosi Coronarica". Circulation. 90 (6): 2710–5. doi:10.1161/01.cir.90.6.2710. PMID 7994812.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.