Cangrelor
 | |
| Names | |
|---|---|
| Trade names | Kengreal, Kengrexal, Canreal |
| Other names | AR-C69931MX |
IUPAC name
| |
| Clinical data | |
| Drug class | P2Y12 inhibitor[1] |
| Main uses | Those undergoing percutaneous coronary intervention (PCI)[2] |
| Side effects | Bleeding, shortness of breath[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | Intravenous |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 100% (IV) |
| Protein binding | ~97–98%. |
| Metabolism | Rapid deactivation in the circulation (independent of CYP system) |
| Elimination half-life | ~3–6 minutes |
| Excretion | Kidney (58%), Bile duct (35%) |
| Chemical and physical data | |
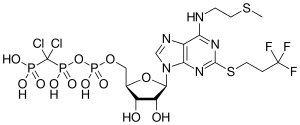
| Formula | C17H25Cl2F3N5O12P3S2 |
| Molar mass | 776.35 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Cangrelor, sold under the brand name Kengreal among others, is a medication used in those undergoing percutaneous coronary intervention (PCI) to prevent blood clots.[2] It is used with aspirin.[2] It is given by injection into a vein.[1]
Common side effects include bleeding and shortness of breath.[2] Other side effects may include allergic reactions.[2] Safety in pregnancy is unclear.[1] It is a P2Y12 inhibitor which blocks platelets from sticking together.[1][2]
Cangrelor was approved for medical use in the United States and Europe in 2015.[1][2] In the United Kingdom 50 mg costs the NHS about £250 as of 2021.[3] This amount in the United States costs about 830 USD.[4]
Medical use
According to recent phase 3 randomized trials, a cangrelor–clopidogrel combination is safe and has been found to be more effective than standard clopidogrel treatment at reducing ischemic events in the heart, without increasing major bleeding in the treatment of stenotic coronary arteries.[5] The advantages of this drug combination are most prominent in patients with myocardial infarction.[5]
Available antiplatelet drugs have delayed onset and offset of action.[5] Since cangrelor’s effects are immediate and quickly reversed, it is a more desirable drug for elective treatment of stenotic coronary arteries, high risk acute coronary syndromes treated with immediate coronary stenting, and for bridging those surgery patients who require P2Y12 inhibition.[5]
Current evidence regarding cangrelor therapy is limited by the lack studies assessing cangrelor administration in conjunction with either prasugrel or ticagrelor.[5]
Recently, it's been approved for adults undergoing percutaneous coronary intervention (PCI). [6]
Poor interim results led to the abandonment of the two CHAMPION clinical trials in mid-2009.[7] The BRIDGE study, for short term use prior to surgery, continues.[8] The CHAMPION PHOENIX trial was a randomized study of over 11,000 patients published in 2013. It found usefulness of cangrelor in people getting cardiac stents. Compared with clopidogrel given around the time of stenting, intravenous cangrelor reduced the rate of stent thrombosis and myocardial infarction.[9] Reviewers have questioned the methodology of the trial.[10]
Dosage
It is given at an initial dose of 30 micrograms per kilogram followed by an infusion at a rate of 4 micrograms per kilogram per minute.[2] It is continued for at least 2 hours following the procedure.[2] Medications by mouth such as clopidogrel, ticagrelor, or prasugrel are than used.[2]
Side effects
Despite fewer bleeding events during cardiac surgery, cangrelor carries the risk of potential autoimmune reactions manifesting as breathlessness.[11] Potential mechanisms for dyspnea following cangrelor treatment include: repeated binding and unbinding cycles, impaired platelet turnover, and lung sequestration or apoptosis of overloaded destructive platelets.[11] The dyspnea risks following cangrelor treatment, suggest a common mechanism linking transfusion-related acute lung injury, dyspnea, and reversible platelet inhibition.[11]
The risk of breathlessness after intravenous cangrelor is smaller when compared with other reversible platelet P2Y12 receptor inhibitors, however, it is still significantly higher when compared to irreversible oral antiplatelet drugs or intravenous glycoprotein IIb/IIIa inhibitors; which do not increase the incidence of breathlessness at all.[11]
Pharmacology
Cangrelor is a high-affinity, reversible inhibitor of P2Y12 receptors that causes almost complete inhibition of ADP-induced platelet aggregate.[12] It is a modified ATP derivative stable to enzymatic degradation.[12] It does not require metabolic conversion to an active metabolite. This allows cangrelor’s immediate effect after infusion,[12] and the therapeutic effects can be maintained with continuous infusion.[13] The pharmacokinetics of cangrelor has allow it to rapidly achieve steady-state concentrations with a clearance of 50 L/h and a half-life of 2.6 to 3.3 minutes. Cessation of its administration is associated with rapid removal, and normal platelet function is restored within 1 hour.[12][13]
Unlike clopidogrel, which is a prodrug, cangrelor is an active drug not requiring metabolic conversion.
References
- 1 2 3 4 5 "Cangrelor Monograph for Professionals". Drugs.com. Archived from the original on 21 January 2021. Retrieved 29 December 2021.
- 1 2 3 4 5 6 7 8 9 10 11 "Kengrexal". Archived from the original on 17 April 2021. Retrieved 29 December 2021.
- ↑ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 225. ISBN 978-0857114105.
- ↑ "Kengreal Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 17 April 2021. Retrieved 29 December 2021.
- 1 2 3 4 5 Kubica J, Kozinski M, Navarese EP, Tantry U, Kubica A, Siller-Matula JM, et al. (May 2014). "Cangrelor: an emerging therapeutic option for patients with coronary artery disease". Current Medical Research and Opinion. 30 (5): 813–28. doi:10.1185/03007995.2014.880050. PMID 24393016. S2CID 30451326.
- ↑ "FDA approves new antiplatelet drug used during heart procedure". FDA News Release. U.S. Food and Drug Administration. 22 June 2015. Archived from the original on 23 June 2015.
- ↑ "CHAMPION Trials With Cangrelor Stopped for Lack of Efficacy". Archived from the original on 2016-06-12. Retrieved 2021-10-26.
- ↑ Napodano J (May 13, 2009). "What Cangrelor Failure Means to Medicines". Seeking Alpha. Archived from the original on March 8, 2016. Retrieved October 26, 2021.
- ↑ Bhatt DL, Stone GW, Mahaffey KW, Gibson CM, Steg PG, Hamm CW, et al. (April 2013). "Effect of platelet inhibition with cangrelor during PCI on ischemic events". The New England Journal of Medicine. 368 (14): 1303–13. doi:10.1056/NEJMoa1300815. PMID 23473369.
- ↑ Lange RA, Hillis LD (April 2013). "The duel between dual antiplatelet therapies". The New England Journal of Medicine. 368 (14): 1356–7. doi:10.1056/NEJMe1302504. PMID 23473370.
- 1 2 3 4 Serebruany VL, Sibbing D, DiNicolantonio JJ (2014). "Dyspnea and reversibility of antiplatelet agents: ticagrelor, elinogrel, cangrelor, and beyond". Cardiology. 127 (1): 20–4. doi:10.1159/000354876. PMID 24192670. S2CID 207707382.
- 1 2 3 4 Angiolillo DJ, Capranzano P (August 2008). "Pharmacology of emerging novel platelet inhibitors". American Heart Journal. 156 (2 Suppl): S10-5. doi:10.1016/j.ahj.2008.06.004. PMID 18657681.
- 1 2 Bhatt DL, Stone GW, Mahaffey KW, Gibson CM, Steg PG, Hamm CW, et al. (April 2013). "Effect of platelet inhibition with cangrelor during PCI on ischemic events". The New England Journal of Medicine. 368 (14): 1303–13. doi:10.1056/nejmoa1300815. PMID 23473369.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- "Cangrelor tetrasodium". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-10-26. Retrieved 2021-10-26.