Topiroxostat
 | |
| Clinical data | |
|---|---|
| Trade names | Topiloric, Uriadec |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
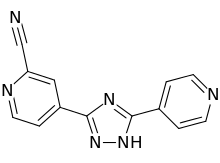
| Formula | C13H8N6 |
| Molar mass | 248.249 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Topiroxostat (INN; trade names Topiloric, Uriadec) is a drug for the treatment of gout and hyperuricemia.[1] It was approved for use in Japan in June 2013.[1]
Topiroxostat is a xanthine oxidase inhibitor which reduces serum urate levels.[2]
References
- 1 2 "New Drugs FY2013" (PDF). Pharmaceuticals and Medical Devices Agency, Japan. Archived from the original (PDF) on 2014-02-22.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Hosoya T, Ohno I, Nomura S, Hisatome I, Uchida S, Fujimori S, et al. (December 2014). "Effects of topiroxostat on the serum urate levels and urinary albumin excretion in hyperuricemic stage 3 chronic kidney disease patients with or without gout". Clinical and Experimental Nephrology. 18 (6): 876–84. doi:10.1007/s10157-014-0935-8. PMC 4271138. PMID 24448692.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.