Caffeine citrate
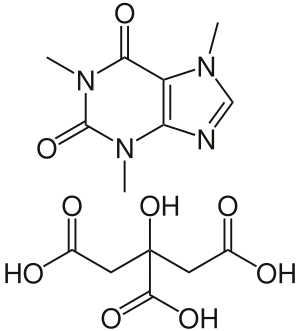 | |
| Names | |
|---|---|
| Trade names | Cafcit, others |
IUPAC name
| |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | by mouth, i.v. |
| Defined daily dose | not established[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Chemical and physical data | |
| Formula | C14H18N4O9 |
| Molar mass | 386.317 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Caffeine citrate, sold under the trade name Cafcit among others, is a medication used to treat a lack of breathing in premature babies.[2] Specifically it is given to babies who are born at less than 35 weeks or weigh less than 2 kilograms (4.4 lb) once other causes are ruled out.[3] It is given by mouth or slow injection into a vein.[2]
Side effects can include problems feeding, increased heart rate, low blood sugar, necrotizing enterocolitis, and kidney problems.[3][2] Testing blood caffeine levels is occasionally recommended.[2] It is a citric acid salt of caffeine.[4] Caffeine citrate is in the xanthine family of medication.[3] It works by stimulating the respiratory centers in the brain.[2]
Caffeine was discovered in 1819.[5] It is on the World Health Organization's List of Essential Medicines.[6] In the United Kingdom a 10 mg vial costs £4.90.[7] The intravenous form may also be taken by mouth.[7]
Medical uses
Caffeine citrate is generally the preferred treatment for apnea of prematurity.[3] It has fewer side effects as compared to theophylline.[3]
Caffeine improves airway function in asthma, increasing forced expiratory volume (FEV1) by 5% to 18%, with this effect lasting for up to four hours.[8]
Dosage
The defined daily dose is not established.[1]
Mechanism
In method of action, the preparation is exactly identical to that of caffeine base as the citrate counter ion dissociates in water. Doses of caffeine citrate, due to the added weight of the citrate moiety, are understandably higher than with caffeine base, i.e., it takes a larger dose to get the same amount of caffeine.[7] The ratio of therapeutic doses of caffeine base to its citrate salt is typically 1:2.[7] Dosing should therefore be clearly distinguished.[7]
History
In June 2020, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) recommended the approval of Gencebok.[9]
Manufacture
The drug is prepared by combining anhydrous caffeine with citric acid monohydrate and sodium citrate dihydrate.
References
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 1 July 2021. Retrieved 16 September 2020.
- 1 2 3 4 5 "Caffeine; Caffeine and Sodium Benzoate Injection; Caffeine Citrate". The American Society of Health-System Pharmacists. Archived from the original on 16 July 2017. Retrieved 8 December 2016.
- 1 2 3 4 5 World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 485. hdl:10665/44053. ISBN 9789241547659.
- ↑ Donn, Steven M.; Sinha, Sunil K. (2012). Manual of Neonatal Respiratory Care. Springer Science & Business Media. p. 457. ISBN 9781461421559. Archived from the original on 2016-12-30.
- ↑ Brown, Nathan (2015). In Silico Medicinal Chemistry: Computational Methods to Support Drug Design. Royal Society of Chemistry. p. 20. ISBN 9781782621638. Archived from the original on 2016-12-29.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- 1 2 3 4 5 Ainsworth, Sean B. (2014). Neonatal Formulary: Drug Use in Pregnancy and the First Year of Life (7 ed.). John Wiley & Sons. p. 120. ISBN 9781118819517. Archived from the original on 2016-12-30.
- ↑ Welsh EJ, Bara A, Barley E, Cates CJ (January 2010). "Caffeine for asthma" (PDF). Cochrane Database Syst Rev (1): CD001112. doi:10.1002/14651858.CD001112.pub2. PMID 20091514. Archived (PDF) from the original on 2019-11-30. Retrieved 2019-09-24.
- ↑ "Gencebok: Pending EC decision". European Medicines Agency (EMA). 25 June 2020. Archived from the original on 27 June 2020. Retrieved 26 June 2020.
External links
| Identifiers: |
|---|
- "Caffeine citrate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2019-12-31. Retrieved 2019-12-31.
- "Caffeine Citrate (CUI C0054436)". NCI Metathesaurus. Archived from the original on 2021-08-28. Retrieved 2019-12-31.