Adenosine
 | |
 | |
| Names | |
|---|---|
| Trade names | Adenocard; Adenocor; Adenic; Adenoco; Adeno-Jec; Adenoscan; Adenosin; Adrekar; Krenosin |
| Other names | SR-96225 |
IUPAC name
| |
| Clinical data | |
| Main uses | Supraventricular tachycardia that do not improve with vagal maneuvers[1] |
| Side effects | Chest pain, feeling faint, shortness of breath, tingling of the skin[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Intravenous[2] |
| Onset of action | <40 sec[2] |
| Duration of action | 1 to 2 min[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | Rapidly cleared from circulation via cellular uptake |
| Protein binding | No |
| Metabolism | Rapidly converted to inosine and adenosine monophosphate |
| Elimination half-life | cleared plasma <30 seconds; half-life <10 seconds |
| Excretion | can leave cell intact or can be degraded to hypoxanthine, xanthine, and ultimately uric acid |
| Chemical and physical data | |
| Formula | C10H13N5O4 |
| Molar mass | 267.245 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Adenosine is a chemical found in mammals which is also used as a medication.[3][4] As a medication it is used to treat or diagnosis certain forms of supraventricular tachycardia that do not improve with vagal maneuvers.[1] It is generally used by rapid injection into a vein.[1] Onset of action is within 40 seconds and effects last for less than 2 minutes.[2]
Common side effects include chest pain, feeling faint, shortness of breath, and tingling of the skin.[1] Serious side effects include a worsening dysrhythmia and low blood pressure.[1] While it may be used during pregnancy, safety is not entirely clear.[5] Adenosine is one of four nucleoside building blocks to RNA.[3] It works by decreasing the frequency by which electrical signals can go through the AV node of the heart.[1]
Adenosine was approved for medical use in the United States in 1989.[1] It; however, has been studied within living systems since the 1920s.[3] Adenosine is avaliable as a generic medication.[6] It is not expensive.[7] In the United Kingdom in 2020 a 6 mg dose costs the NHS about a pound.[6]
Medical uses
Supraventricular tachycardia
In individuals with supraventricular tachycardia (SVT), adenosine is used to help identify and convert the rhythm.[8] This includes any re-entrant arrhythmias that require the AV node for the re-entry, e.g., AV reentrant tachycardia (AVRT), AV nodal reentrant tachycardia (AVNRT). In addition, atrial tachycardia can sometimes be terminated with adenosine. It may be used in SVT with Wolff-Parkinson-White syndrome.[6]
Fast rhythms of the heart that are confined to the atria (e.g., atrial fibrillation, atrial flutter) or ventricles (e.g., monomorphic ventricular tachycardia) and do not involve the AV node as part of the re-entrant circuit are not typically converted by adenosine. However, the ventricular response rate is temporarily slowed with adenosine in such cases.
Because of the effects of adenosine on AV node-dependent SVTs, adenosine is considered a class V antiarrhythmic agent. When adenosine is used to cardiovert an abnormal rhythm, it is normal for the heart to enter ventricular asystole for a few seconds. This can be disconcerting to a normally conscious people, and is associated with angina-like sensations in the chest.[9]
Nuclear stress test
Adenosine is used as an adjunct to thallium (TI 201) or technetium (Tc99m) myocardial perfusion scintigraphy (nuclear stress test) in people unable to undergo adequate stress testing with exercise.[10]
Dosage
When given for the evaluation or treatment of a supraventricular tachycardia (SVT), the initial dose is 6 mg which may be followed by a dose of 12 mg.[1] In children it is used at a dose of 0.3 mg/kg.[1] In those who have had a heart transplant half the typical dose is used.[6]
It is given as a rapid injection into a vein. Due to adenosine's extremely short half-life, the IV line is started as proximal (near) to the heart as possible, such as the antecubital fossa. The IV push is often followed with a flush of 10–20 mL of normal saline. If this has no effect (i.e., no evidence of transient AV block), a dose of 12 mg can be given 1–2 minutes after the first dose. The either 6 or 12 mg dose of adenosine can also be mixed in a 10 ml syringe of normal saline and than pushed.[11]
When given to dilate the arteries, such as in a "stress test", the dosage is typically 0.14 mg/kg/min, administered for 4 or 6 minutes, depending on the protocol.
The recommended dose may be increased in people on theophylline, since methylxanthines prevent binding of adenosine at receptor sites. The dose is often decreased in people on dipyridamole and diazepam because adenosine potentiates the effects of these drugs. The recommended dose is also reduced by half in people presenting congestive heart failure, myocardial infarction, shock, hypoxia, and/or chronic liver disease or chronic kidney disease, and in the elderly.
Side effects
Common side effects include chest pain, feeling faint, shortness of breath, and tingling of the skin.[1] Serious side effects include a worsening dysrhythmia and low blood pressure.[1]
Pregnancy and breastfeeding
While it may be used during pregnancy, safety is not entirely clear.[5] Large doses may result in harm to the baby.[6] While it is not known if adenosine enters breastmilk, this is believed to be unlikely as it is so rapidly broken down.[6]
Contraindications
Common contraindications for adenosine include
- Asthma, traditionally considered an absolute contraindication. This is being contended and it is now considered a relative contraindication (however, selective adenosine antagonists are being investigated for use in treatment of asthma)[12]
Interactions
Dipyridamole potentiates the action of adenosine, requiring the use of lower doses.
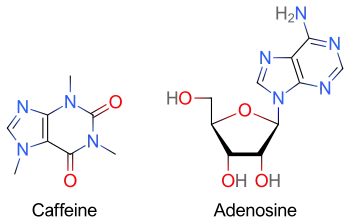
Methylxanthines (e.g. caffeine, found in coffee, theophylline in tea, or theobromine, found in chocolate) have a purine structure and bind to some of the same receptors as adenosine.[13] Methylxanthines act as competitive antagonists of adenosine and can blunt its pharmacological effects.[14] Individuals taking large quantities of methylxanthines may require increased doses of adenosine.
Mechanism of action
Adenosine consists of an adenine attached to a ribose via a β-N9-glycosidic bond.
Adenosine is an endogenous purine nucleoside that modulates many physiological processes. Cellular signaling by adenosine occurs through four known adenosine receptor subtypes (A1, A2A, A2B, and A3).[15]
Extracellular adenosine concentrations from normal cells are approximately 300 nM; however, in response to cellular damage (e.g., in inflammatory or ischemic tissue), these concentrations are quickly elevated (600–1,200 nM). Thus, in regard to stress or injury, the function of adenosine is primarily that of cytoprotection preventing tissue damage during instances of hypoxia, ischemia, and seizure activity. Activation of A2A receptors produces a constellation of responses that in general can be classified as anti-inflammatory.[16] Enzymatic production of adenosine can be anti-inflammatory or immunosuppressive.[17][18][19][20]
When it is administered intravenously, adenosine causes transient heart block in the atrioventricular (AV) node. This is mediated via the A1 receptor, inhibiting adenylyl cyclase, reducing cAMP and so causing cell hyperpolarization by increasing K+ efflux via inward rectifier K+ channels, subsequently inhibiting Ca2+ current.[21] It also causes endothelial-dependent relaxation of smooth muscle as is found inside the artery walls. This causes dilation of the "normal" segments of arteries, i.e. where the endothelium is not separated from the tunica media by atherosclerotic plaque. This feature allows physicians to use adenosine to test for blockages in the coronary arteries, by exaggerating the difference between the normal and abnormal segments.
The administration of adenosine also reduces blood flow to coronary arteries past the occlusion. Other coronary arteries dilate when adenosine is administered while the segment past the occlusion is already maximally dilated, which is a process called coronary steal. This leads to less blood reaching the ischemic tissue, which in turn produces the characteristic chest pain.
Adenosine receptors
All adenosine receptor subtypes (A1, A2A, A2B, and A3) are G-protein-coupled receptors. The four receptor subtypes are further classified based on their ability to either stimulate or inhibit adenylate cyclase activity. The A1 receptors couple to Gi/o and decreases cAMP levels, while the A2 adenosine receptors couple to Gs, which stimulates adenylate cyclase activity. In addition, A1 receptors couple to Go, which has been reported to mediate adenosine inhibition of Ca2+ conductance, whereas A2B and A3 receptors also couple to Gq and stimulate phospholipase activity. Researchers at Cornell University have recently shown adenosine receptors to be key in opening the blood-brain barrier (BBB). Mice dosed with adenosine have shown increased transport across the BBB of amyloid plaque antibodies and prodrugs associated with Parkinson's disease, Alzheimer's, multiple sclerosis, and cancers of the central nervous system.[22]
Growth hormone
Adenosine is an endogenous agonist of the ghrelin/growth hormone secretagogue receptor.[23] However, while it is able to increase appetite, unlike other agonists of this receptor, adenosine is unable to induce the secretion of growth hormone and increase its plasma levels.[23]
Metabolism
Adenosine used as a second messenger can be the result of de novo purine biosynthesis via adenosine monophosphate (AMP), though it is possible other pathways exist.[24]
When adenosine enters the circulation, it is broken down by adenosine deaminase, which is present in red blood cells and the vessel wall.
Dipyridamole, an inhibitor of adenosine nucleoside transporter, allows adenosine to accumulate in the blood stream. This causes an increase in coronary vasodilatation.
Adenosine deaminase deficiency is a known cause of immunodeficiency.
Its derivatives include the energy carriers adenosine mono-, di-, and triphosphate. Cyclic adenosine monophosphate (cAMP) is pervasive in signal transduction. Adenosyl (Ad) is the radical formed by removal of the 5'-hydroxy (OH) group. Ad is found in vitamin B12 and the radical SAM enzymes.[25]
Research
Viruses
The adenosine analog NITD008 has been reported to directly inhibit the recombinant RNA-dependent RNA polymerase of the dengue virus by terminating its RNA chain synthesis. This interaction suppresses peak viremia and rise in cytokines and prevents lethality in infected animals, raising the possibility of a new treatment for this flavivirus.[26] The 7-deaza-adenosine analog has been shown to inhibit the replication of the hepatitis C virus.[27] BCX4430 is protective against Ebola and Marburg viruses.[28] Such adenosine analogs are potentially clinically useful since they can be taken orally.
Inflammation
Adenosine is believed to be an anti-inflammatory agent at the A2A receptor.[29][30] Topical treatment of adenosine to foot wounds in diabetes mellitus has been shown in lab animals to drastically increase tissue repair and reconstruction. Topical administration of adenosine for use in wound-healing deficiencies and diabetes mellitus in humans is currently under clinical investigation.
Methotrexate's anti-inflammatory effect may be due to its stimulation of adenosine release.[31]
Central nervous system
In general, adenosine has an inhibitory effect in the central nervous system (CNS). Caffeine's stimulatory effects are credited primarily (although not entirely) to its capacity to block adenosine receptors, thereby reducing the inhibitory tonus of adenosine in the CNS. This reduction in adenosine activity leads to increased activity of the neurotransmitters dopamine and glutamate.[32] Experimental evidence suggests that adenosine and adenosine agonists can activate Trk receptor phosphorylation through a mechanism that requires the adenosine A2A receptor.[33]
Hair
Adenosine has been shown to promote thickening of hair on people with thinning hair.[34][35] A 2013 study compared topical adenosine with minoxidil in male androgenetic alopecia, finding it was not superior to minoxidil and further trials were needed.[36]
Sleep
The principal component of marijuana, delta-9-tetrahydrocannabinol (THC) and the endocannabinoid anandamide (AEA) induce sleep in rats by increasing adenosine levels in the basal forebrain. They also significantly increase slow-wave sleep during the third hour, mediated by CB1 receptor activation. These findings identify a potential therapeutic use of cannabinoids to induce sleep in conditions where sleep may be severely attenuated.[37]
Infarct size determination
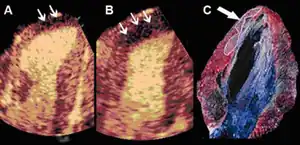
Adenosine infusion, can increase perfusional defect, as a result per an animal study indicates that "a better correlation between the echocardiographic measurements and the necrotic area determined by tissue staining". [38]
See also
- Adenosine receptor
- Adenosine reuptake inhibitor
- List of growth hormone secretagogues
References
- 1 2 3 4 5 6 7 8 9 10 11 12 "Adenosine". The American Society of Health-System Pharmacists. Archived from the original on December 23, 2014. Retrieved Sep 27, 2020.
- 1 2 3 4 Aehlert, Barbara J. (2015). ECGs Made Easy - E-Book. Elsevier Health Sciences. p. 116. ISBN 978-0-323-39150-4. Archived from the original on 27 August 2021. Retrieved 29 September 2020.
- 1 2 3 Adenosine Receptors in Neurology and Psychiatry. Academic Press. 2014. p. XV. ISBN 978-0-12-801318-2. Archived from the original on 2021-08-27. Retrieved 2020-09-28.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 18–. ISBN 978-3-88763-075-1. Archived from the original on 2016-05-04. Retrieved 2016-01-05.
- 1 2 "Adenosine Use During Pregnancy". Drugs.com. Archived from the original on 5 December 2020. Retrieved 28 September 2020.
- 1 2 3 4 5 6 BNF 79. London: Pharmaceutical Press. March 2020. p. 110. ISBN 978-0857113658.
- ↑ Hitchings, Andrew; Lonsdale, Dagan; Burrage, Daniel; Baker, Emma (2019). The Top 100 Drugs: Clinical Pharmacology and Practical Prescribing (2nd ed.). Elsevier. pp. 34–35. ISBN 978-0-7020-7442-4. Archived from the original on 2021-05-22. Retrieved 2021-11-09.
- ↑ Mitchell J, Lazarenko G (November 2008). "Wide QRS complex tachycardia. Diagnosis: Supraventricular tachycardia with aberrant conduction; intravenous (IV) adenosine". CJEM. 10 (6): 572–73, 581. PMID 19000353.
- ↑ Pijls, Nico H. J.; Bernard De Bruyne (2000). Coronary Pressure. Springer. ISBN 0-7923-6170-9.
- ↑ O'Keefe, JH; Bateman, TM; Silverstri, R; et al. (1992). "Safety and diagnostic accuracy of adenosine thallium-201 scintigraphy in patients unable to exercise and those with left bundle branch block". Am. Heart J. 124 (3): 614–21. doi:10.1016/0002-8703(92)90268-z. PMID 1514488.
- ↑ Miyawaki, IA; Gomes, C; Caporal S Moreira, V; R Marques, I; A F de Souza, I; H A Silva, C; Riceto Loyola Júnior, JE; Huh, K; McDowell, M; Padrao, EMH; Tichauer, MB; Gibson, CM (July 2023). "The Single-Syringe Versus the Double-Syringe Techniques of Adenosine Administration for Supraventricular Tachycardia: A Systematic Review and Meta-Analysis". American journal of cardiovascular drugs : drugs, devices, and other interventions. 23 (4): 341–353. doi:10.1007/s40256-023-00581-w. PMID 37162718.
- ↑ Brown RA, Spina D, Page CP (March 2008). "Adenosine receptors and asthma". Br. J. Pharmacol. 153 Suppl 1 (S1): S446–56. doi:10.1038/bjp.2008.22. PMC 2268070. PMID 18311158.
- ↑ "Caffeine". Chemistry Explained. Archived from the original on 2011-05-20. Retrieved 2011-04-07.
- ↑ "Vitamin B4". R&S Pharmchem. April 2011. Archived from the original on 2011-07-15.
- ↑ Haskó G, Linden J, Cronstein B, Pacher P (September 2008). "Adenosine receptors: therapeutic aspects for inflammatory and immune diseases". Nat Rev Drug Discov. 7 (9): 759–70. doi:10.1038/nrd2638. PMC 2568887. PMID 18758473.
- ↑ Haskó, G (January 2004). "Adenosine: an endogenous regulator of innate immunity". Trends in Immunology. 25 (1): 33–39. doi:10.1016/j.it.2003.11.003. PMID 14698282.
- ↑ Sek K, Mølck C, Stewart GD, Kats L (2018). "Targeting Adenosine Receptor Signaling in Cancer Immunotherapy". International Journal of Molecular Sciences. 19 (12): 3837. doi:10.3390/ijms19123837. PMC 6321150. PMID 30513816.
- ↑ Sek K, Mølck C, Stewart GD, Kats L (2018). "Targeting Adenosine Receptor Signaling in Cancer Immunotherapy". International Journal of Molecular Sciences. 19 (12): 3837. doi:10.3390/ijms19123837. PMC 6321150. PMID 30513816.
- ↑ Konen JM, Fradette JJ, Gibbons DL (2019). "The Good, the Bad and the Unknown of CD38 in the Metabolic Microenvironment and Immune Cell Functionality of Solid Tumors". Cells. 9 (1): 52. doi:10.3390/cells9010052. PMC 7016859. PMID 31878283.
- ↑ Antonioli L, Pacher P, Vizi ES, Haskó G (2013). "CD39 and CD73 in immunity and inflammation". Trends in Molecular Medicine. 19 (6): 355–367. doi:10.1016/j.molmed.2013.03.005. PMC 3674206. PMID 23601906. Archived from the original on 2020-07-28. Retrieved 2020-07-28.
- ↑ Katzung, Bertram (2012). Basic & Clinical Pharmacology (12th ed.). McGraw Hill. p. 245. ISBN 978-0-07-176402-5.
- ↑ Carman, A. J.; Mills, J. H.; Krenz, A; Kim, D. G.; Bynoe, M. S. (2011). "Adenosine receptor signaling modulates permeability of the blood-brain barrier". J. Neurosci. 31 (37): 13272–80. doi:10.1523/JNEUROSCI.3337-11.2011. PMC 3328085. PMID 21917810.
- 1 2 Claude Kordon; I. Robinson; Jacques Hanoune; R. Dantzer (2012). Brain Somatic Cross-Talk and the Central Control of Metabolism. Springer Science & Business Media. pp. 42–. ISBN 978-3-642-18999-9. Archived from the original on 2016-05-10. Retrieved 2016-01-05.
- ↑ Miller-Patrick K, Vincent DL, Early RJ, et al. (1993). "Effects of the purine biosynthesis pathway inhibitors azaserine, hadacidin, and mycophenolic acid on the developing ovine corpus luteum". Chin J Physiol. 36 (4): 245–52. PMID 8020339.
- ↑ Nelson, David L.; Cox, Michael M. (2005), Principles of Biochemistry (4th ed.), New York: W. H. Freeman, ISBN 0-7167-4339-6
- ↑ Yin, Z; Chen, YL; Schul, W; Wang, QY; Gu, F; Duraiswamy, J; Reddy Kondreddi, R; Niyomrattanakit, P; Lakshminarayana, SB; Goh, A; Xu, HY; Liu, W; Liu, B; Lim, JY; Ng, CY; Qing, M; Lim, CC; Yip, A; Wang, G; Chan, WL; Tan, HP; Lin, K; Zhang, B; Zou, G; Bernard, KA; Garrett, C; Beltz, K; Dong, M; Weaver, M; He, H; Pichota, A; Dartois, V; Keller, TH; Shi, PY (2009). "An adenosine nucleoside inhibitor of dengue virus". Proc Natl Acad Sci U S A. 106 (48): 20435–39. Bibcode:2009PNAS..10620435Y. doi:10.1073/pnas.0907010106. PMC 2787148. PMID 19918064.
- ↑ Olsen, DB; Eldrup, AB; Bartholomew, L; Bhat, B; Bosserman, MR; Ceccacci, A; Colwell, LF; Fay, JF; Flores, OA; Getty, K. L.; Grobler, J. A.; Lafemina, R. L.; Markel, E. J.; Migliaccio, G.; Prhavc, M.; Stahlhut, M. W.; Tomassini, J. E.; MacCoss, M.; Hazuda, D. J.; Carroll, S. S. (2004). "A 7-Deaza-Adenosine Analog Is a Potent and Selective Inhibitor of Hepatitis C Virus Replication with Excellent Pharmacokinetic Properties". Antimicrobial Agents and Chemotherapy. 48 (10): 3944–53. doi:10.1128/AAC.48.10.3944-3953.2004. PMC 521892. PMID 15388457.
- ↑ Warren, T. K.; Wells, J.; Panchal, R. G.; Stuthman, K. S.; Garza, N. L.; Van Tongeren, S. A.; Dong, L.; Retterer, C. J.; Eaton, B. P.; Pegoraro, G.; Honnold, S.; Bantia, S.; Kotian, P.; Chen, X.; Taubenheim, B. R.; Welch, L. S.; Minning, D. M.; Babu, Y. S.; Sheridan, W. P.; Bavari, S. (2014). "Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430". Nature. 508 (7496): 402–05. Bibcode:2014Natur.508..402W. doi:10.1038/nature13027. PMC 7095208. PMID 24590073. Archived from the original on 2019-12-07. Retrieved 2019-12-17.
- ↑ Nakav S, Chaimovitz C, Sufaro Y (2008). Bozza P (ed.). "Anti-Inflammatory Preconditioning by Agonists of Adenosine A1 Receptor". PLOS ONE. 3 (5): e2107. Bibcode:2008PLoSO...3.2107N. doi:10.1371/journal.pone.0002107. PMC 2329854. PMID 18461129.
- ↑ Trevethick MA, Mantell SJ, Stuart EF, Barnard A, Wright KN, Yeadon M (October 2008). "Treating lung inflammation with agonists of the adenosine A2A receptor: promises, problems and potential solutions". Br. J. Pharmacol. 155 (4): 463–74. doi:10.1038/bjp.2008.329. PMC 2579671. PMID 18846036.
- ↑ Cronstein B (2010). "How does methotrexate suppress inflammation?". Clin Exp Rheumatol. 28 (5 Suppl 61): S21–23. PMID 21044428.
- ↑ Solinas, M; Ferré, S; You, Z. B; Karcz-Kubicha, M; Popoli, P; Goldberg, S. R (2002). "Caffeine induces dopamine and glutamate release in the shell of the nucleus accumbens". The Journal of Neuroscience. 22 (15): 6321–24. doi:10.1523/JNEUROSCI.22-15-06321.2002. PMC 6758129. PMID 12151508.
- ↑ Lee, FS; Chao, MV; Lee (March 2001). "Activation of Trk neurotrophin receptors in the absence of neurotrophins". PNAS. 98 (6): 3555–60. Bibcode:2001PNAS...98.3555L. doi:10.1073/pnas.061020198. PMC 30691. PMID 11248116.
- ↑ Oura, H; Iino, M; Nakazawa, Y; Tajima, M; Ideta, R; Nakaya, Y; Arase, S; Kishimoto, J (December 2008). "Adenosine increases anagen hair growth and thick hairs in Japanese women with female pattern hair loss: a pilot, double-blind, randomized, placebo-controlled trial". The Journal of Dermatology. 35 (12): 763–67. doi:10.1111/j.1346-8138.2008.00564.x. PMID 19239555.
- ↑ Hwang, KA; Hwang, YL; Lee, MH; Kim, NR; Roh, SS; Lee, Y; Kim, CD; Lee, JH; Choi, KC (February 2012). "Adenosine stimulates growth of dermal papilla and lengthens the anagen phase by increasing the cysteine level via fibroblast growth factors 2 and 7 in an organ culture of mouse vibrissae hair follicles". International Journal of Molecular Medicine. 29 (2): 195–201. doi:10.3892/ijmm.2011.817. PMID 22020741.
- ↑ Faghihi, G; Iraji, F; Rajaee Harandi, M; Nilforoushzadeh, M. A.; Askari, G (2013). "Comparison of the efficacy of topical minoxidil 5% and adenosine 0.75% solutions on male androgenetic alopecia and measuring patient satisfaction rate". Acta Dermatovenerol Croat. 21 (3): 155–59. PMID 24183218.
- ↑ Murillo-Rodriguez, Eric; Blanco-Centurion, Carlos; Sanchez, Cristina; Daniele, Piomelli; Shiromani, Priyattam J. (2003). "Anandamide Enhances Extracellular Levels of Adenosine and Induces Sleep: An In Vivo Microdialysis Study". Sleep. 26 (8): 943–47. doi:10.1093/sleep/26.8.943. ISSN 0161-8105. PMID 14746372. Archived from the original on 2018-06-29. Retrieved 2018-06-29.
- ↑ Dourado, Paulo Magno Martins; Tsutsui, Jeane Mike; Chagas, Antonio Carlos Palandri; Sbano, João César Nunes; Aiello, Vera demarchi; da Luz, Protásio Lemos; Mathias Jr, Wilson; Ramires, Jose AF (8 February 2006). "Value of adenosine infusion for infarct size determination using real-time myocardial contrast echocardiography". Cardiovascular Ultrasound. 4: 10. doi:10.1186/1476-7120-4-10. ISSN 1476-7120. Archived from the original on 9 October 2021. Retrieved 8 October 2021.
External links
| Identifiers: |
|---|