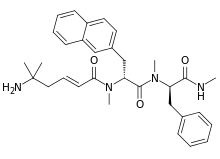
Tabimorelin
 | |
| Clinical data | |
|---|---|
| Other names | ((2E)-5-amino-5-methylhex-2-enoic acid N-methyl-N-((1R)-1-(N-methyl-N-((1R)-1-(methylcarbamoyl)-2-phenylethyl)carbamoyl)-2-(2-naphthyl)ethyl)amide) |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C32H40N4O3 |
| Molar mass | 528.697 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Tabimorelin (INN) (developmental code name NN-703) is a drug which acts as a potent, orally-active agonist of the ghrelin/growth hormone secretagogue receptor (GHSR) and growth hormone secretagogue, mimicking the effects of the endogenous peptide agonist ghrelin as a stimulator of growth hormone (GH) release. It was one of the first GH secretagogues developed and is largely a modified polypeptide, but it is nevertheless orally-active in vivo.[1] Tabimorelin produced sustained increases in levels of GH and insulin-like growth factor 1 (IGF-1), along with smaller transient increases in levels of other hormones such as adrenocorticotropic hormone (ACTH), cortisol, and prolactin.[2][3] However actual clinical effects in adults with growth hormone deficiency were limited, with only the most severely GH-deficient patients showing significant benefit,[4] and tabimorelin was also found to act as a CYP3A4 inhibitor which could cause it to have undesirable interactions with other drugs.[5]
See also
References
- ↑ Hansen BS, Raun K, Nielsen KK, Johansen PB, Hansen TK, Peschke B, et al. (August 1999). "Pharmacological characterisation of a new oral GH secretagogue, NN703". European Journal of Endocrinology. 141 (2): 180–9. doi:10.1530/eje.0.1410180. PMID 10427162.
- ↑ Zdravkovic M, Søgaard B, Ynddal L, Christiansen T, Agersø H, Thomsen MS, et al. (August 2000). "The pharmacokinetics, pharmacodynamics, safety and tolerability of a single dose of NN703, a novel orally active growth hormone secretagogue in healthy male volunteers". Growth Hormone & IGF Research. 10 (4): 193–8. doi:10.1054/ghir.2000.0152. PMID 11032702.
- ↑ Zdravkovic M, Christiansen T, Eliot L, Agersoe H, Thomsen MS, Falch JF, et al. (February 2001). "The pharmacokinetics, pharmacodynamics, safety and tolerability following 7 days daily oral treatment with NN703 in healthy male subjects". Growth Hormone & IGF Research. 11 (1): 41–8. doi:10.1054/ghir.2000.0188. PMID 11437473.
- ↑ Svensson J, Monson JP, Vetter T, Hansen TK, Savine R, Kann P, et al. (May 2003). "Oral administration of the growth hormone secretagogue NN703 in adult patients with growth hormone deficiency". Clinical Endocrinology. 58 (5): 572–80. doi:10.1046/j.1365-2265.2003.01754.x. PMID 12699438. S2CID 33510538.
- ↑ Zdravkovic M, Olsen AK, Christiansen T, Schulz R, Taub ME, Thomsen MS, et al. (February 2003). "A clinical study investigating the pharmacokinetic interaction between NN703 (tabimorelin), a potential inhibitor of CYP3A4 activity, and midazolam, a CYP3A4 substrate". European Journal of Clinical Pharmacology. 58 (10): 683–8. doi:10.1007/s00228-002-0539-1. PMID 12610745. S2CID 22546247.