Octreotide
 | |
 | |
| Names | |
|---|---|
| Trade names | Sandostatin, Bynfezia Pen, Mycapssa, others |
IUPAC name
| |
| Clinical data | |
| Drug class | Somatostatin[1] |
| Main uses | Carcinoid syndrome, acromegaly, congenital hyperinsulinism, gastrointestinal bleeding due to esophageal varices[1] |
| Side effects | Nausea, slow heart rate, abdominal discomfort[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Subcutaneous, intramuscular, intravenous, by mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a693049 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 60% (IM), 100% (SC) |
| Protein binding | 40–65% |
| Metabolism | Liver |
| Elimination half-life | 1.7–1.9 hours |
| Excretion | Urine (32%) |
| Chemical and physical data | |
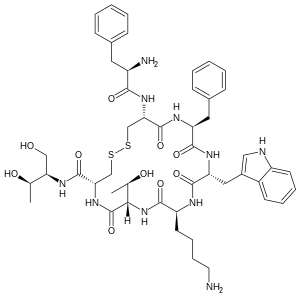
| Formula | C49H66N10O10S2 |
| Molar mass | 1019.25 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Octreotide, sold under the brand name Sandostatin among others, is a medication used to treat carcinoid syndrome, acromegaly, congenital hyperinsulinism, and gastrointestinal bleeding due to esophageal varices.[1][2] It can be given by injection into a vein or muscle.[1] The immediate release form may be effective up to 12 hours.[1]
Common side effects include nausea, a slow heart rate, and abdominal discomfort.[1] Other side effects may include low or high blood sugar, gall bladder problems, and anaphylaxis.[1] It is unclear if use during pregnancy is safe.[3] Use is not recommended when breastfeeding.[4] It mimics the effects of natural somatostatin and inhibits the release of growth hormone, serotonin, insulin, and glucagon.[1]
Octreotide was first made in 1979, by the chemist Wilfried Bauer.[5] It is on the World Health Organization's List of Essential Medicines.[6] It was approved for medical use in the United States in 1988.[7] It is available as a generic medication.[4] In the United Kingdom it costs about 3 pounds per 50 ug dose as of 2020.[4] In the United States this amount costs about 5.30 USD as of 2020.[8]
Medical uses

Tumors
Octreotide is used for the treatment of growth hormone producing tumors (acromegaly and gigantism), when surgery is contraindicated, pituitary tumors that secrete thyroid-stimulating hormone (thyrotropinoma), diarrhea and flushing episodes associated with carcinoid syndrome, and diarrhea in people with vasoactive intestinal peptide-secreting tumors (VIPomas). Octreotide is also used in mild cases of glucagonoma when surgery is not an option.[9][10]
Bleeding esophageal varices
Octreotide is often given as an infusion for management of acute hemorrhage from esophageal varices in liver cirrhosis on the basis that it reduces portal venous pressure, though current evidence suggests that this effect is transient and does not improve survival.[11]
The recommended dose for this purpose is often a 50 ug bolus followed by 50 ug per hour for five days.[2]
Radiolabeling

Octreotide is used in nuclear medicine imaging by labeling with indium-111 (Octreoscan) to noninvasively image neuroendocrine and other tumours expressing somatostatin receptors.[12] More recently, it has been radiolabeled with carbon-11[13] as well as gallium-68, enabling imaging with positron emission tomography (PET), which provides higher resolution and sensitivity.
Octreotide can also be labeled with a variety of radionuclides, such as yttrium-90 or lutetium-177, to enable peptide receptor radionuclide therapy (PRRT) for the treatment of unresectable neuroendocrine tumours.
Acromegaly
Octreotide can also be used in the treatment of acromegaly, a disorder of excessive growth hormone (GH). Octreotide, being a somatostatin analog, inhibits the release of GH from the pituitary gland through a process normally involved in negative feedback.
In June 2020, Mycapssa (octreotide) was approved for medical use in the United States with an indication for the long-term maintenance treatment in acromegaly patients who have responded to and tolerated treatment with octreotide or lanreotide.[14][15] Mycapssa is the first and only oral somatostatin analog (SSA) approved by the FDA.[15]
Gastrointestinal fistulae
Octreotide helps in management of the fistula by reducing gastrointestinal secretions and inhibiting gastrointestinal motility, thus controlling and reducing its output. The value in healing intestinal fistulas is yet to be proven and routine use is limited because of the side effects.
Hypoglycemia
Octreotide is also used in the treatment of refractory hypoglycemia in neonates and sulphonylurea-induced hypoglycemia in adults.
Dosage
The dose of octreotide in those with varices and upper gastrointestinal bleeding is an intravenous bolus of 20 mcg to 50 mcg, followed by a continuous infusion at a rate of 25 mcg to 50 mcg per hour.[16]
Contraindications
Octreotide has not been adequately studied for the treatment of children as well as pregnant and lactating women. The drug is given to these groups only if a risk-benefit analysis is positive.[17][18]
Side effects
The most common side effects are headache, hypothyroidism, cardiac conduction changes, gastrointestinal reactions (including cramps, nausea/vomiting and diarrhoea or constipation), gallstones, reduction of insulin release, hyperglycemia[19] or sometimes hypoglycemia, and (usually transient) injection site reactions. Slow heart rate, skin reactions such as pruritus, hyperbilirubinemia, hypothyroidism, dizziness and dyspnoea are also fairly common (more than 1%). Rare side effects include acute anaphylactic reactions, pancreatitis and hepatitis.[17][18]
Some studies reported alopecia in those who were treated by octreotide.[20] Rats which were treated by octreotide experienced erectile dysfunction in a 1998 study.[21]
A prolonged QT interval has been observed, but it is uncertain whether this is a reaction to the drug or the result of an existing illness.[17]
Overdose
Octreotide is useful in overdose management of sulfonylurea type hypoglycemic medications, when recurrent or refractory to parenteral dextrose. Mechanism of action is the suppression of insulin secretion.
Interactions
Octreotide can reduce the intestinal reabsorption of ciclosporin, possibly making it necessary to increase the dose.[22] People with diabetes mellitus might need less insulin or oral antidiabetics when treated with octreotide, as it inhibits glucagon secretion more strongly and for a longer time span than insulin secretion.[17] The bioavailability of bromocriptine is increased;[18] besides being an antiparkinsonian, bromocriptine is also used for the treatment of acromegaly.
Pharmacology
| Identifiers | |
|---|---|
| Symbol | N/A |
| OPM superfamily | 158 |
| OPM protein | 1soc |
Since octreotide resembles somatostatin in physiological activities, it can:
- inhibit secretion of many hormones, such as gastrin, cholecystokinin, glucagon, growth hormone, insulin, secretin, pancreatic polypeptide, TSH, and vasoactive intestinal peptide,
- reduce secretion of fluids by the intestine and pancreas,
- reduce gastrointestinal motility and inhibit contraction of the gallbladder,
- inhibit the action of certain hormones from the anterior pituitary,
- cause vasoconstriction in the blood vessels, and
- reduce portal vessel pressures in bleeding varices.
It has also been shown to produce analgesic effects, most probably acting as a partial agonist at the mu opioid receptor.[23][24]
Pharmacokinetics
Octreotide is absorbed quickly and completely after subcutaneous application. Maximal plasma concentration is reached after 30 minutes. The elimination half-life is 100 minutes (1.7 hours) on average when applied subcutaneously; after intravenous injection, the substance is eliminated in two phases with half-lives of 10 and 90 minutes, respectively.[17][18]
History
Octreotide acetate was approved for use in the United States in 1988.[25][26]
In January 2020, approval of octreotide acetate in the United States was granted to Sun Pharmaceutical under the brand name Bynfezia Pen for the treatment of:[26][27]
- the reduction of growth hormone and insulin-like growth factor 1 (somatomedin C) in adults with acromegaly who have had inadequate response to or cannot be treated with surgical resection, pituitary irradiation, and bromocriptine mesylate at maximally tolerated doses
- severe diarrhea/flushing episodes associated with metastatic carcinoid tumors in adults
- profuse watery diarrhea associated with vasoactive intestinal peptide tumors (VIPomas) in adults
Research
Octreotide has also been used off-label for the treatment of severe, refractory diarrhea from other causes. It is used in toxicology for the treatment of prolonged recurrent hypoglycemia after sulfonylurea and possibly meglitinide overdose. It has also been used with varying degrees of success in infants with nesidioblastosis to help decrease insulin hypersecretion. Several clinical trials have demonstrated the effect of octreotide as acute treatment (abortive agent) in cluster headache, where it has been shown that administration of subcutaneous octreotide is effective when compared with placebo.[28]
Octreotide has also been investigated in people with pain from chronic pancreatitis.[29]
It has been used in the treatment of malignant bowel obstruction.[30]
Octreotide may be used in conjunction with midodrine to partially reverse peripheral vasodilation in the hepatorenal syndrome. By increasing systemic vascular resistance, these drugs reduce shunting and improve renal perfusion, prolonging survival until definitive treatment with liver transplant.[31] Similarly, octreotide can be used to treat refractory chronic hypotension.[32]
While successful treatment has been demonstrated in case reports,[33][34] larger studies have failed to demonstrate efficacy in treating chylothorax.[35]
A small study has shown that octreotide may be effective in the treatment of idiopathic intracranial hypertension.[36][37]
Obesity
Octreotide has been used experimentally to treat obesity, particularly obesity caused by lesions in the hunger and satiety centers of the hypothalamus, a region of the brain central to the regulation of food intake and energy expenditure.[38] The circuit begins with an area of the hypothalamus, the arcuate nucleus, that has outputs to the lateral hypothalamus (LH) and ventromedial hypothalamus (VMH), the brain's feeding and satiety centers, respectively.[39][40] The VMH is sometimes injured by ongoing treatment for acute lymphoblastic leukemia (ALL) or surgery or radiation to treat posterior cranial fossa tumors.[38] With the VMH disabled and no longer responding to peripheral energy balance signals, "Efferent sympathetic activity drops, resulting in malaise and reduced energy expenditure, and vagal activity increases, resulting in increased insulin secretion and adipogenesis."[41] "VMH dysfunction promotes excessive caloric intake and decreased caloric expenditure, leading to continuous and unrelenting weight gain. Attempts at caloric restriction or pharmacotherapy with adrenergic or serotonergic agents have previously met with little or only brief success in treating this syndrome."[38] In this context, octreotide suppresses the excessive release of insulin and may increase its action, thereby inhibiting excessive adipose storage. In a small clinical trial in eighteen pediatric subjects with intractable weight gain following therapy for ALL or brain tumors and other evidence of hypothalamic dysfunction, octreotide reduced body mass index (BMI) and insulin response during glucose tolerance test, while increasing parent-reported physical activity and quality of life (QoL) relative to placebo.[38] In a separate placebo-controlled trial of obese adults without known hypothalamic lesions, obese subjects who received long-acting octreotide lost weight and reduced their BMI compared to subjects receiving placebo; post hoc analysis suggested greater effects in patients receiving the higher dose of the drug, and among "Caucasian subjects having insulin secretion greater than the median of the cohort." "There were no statistically significant changes in QoL scores, body fat, leptin concentration, Beck Depression Inventory, or macronutrient intake", although subjects taking octreotide had higher blood glucose after a glucose tolerance test than those receiving placebo.[42]
See also
- Octreotate, a closely related peptide
References
- 1 2 3 4 5 6 7 8 9 "Octreotide Monograph for Professionals". Drugs.com. Archived from the original on 24 January 2021. Retrieved 25 December 2020.
- 1 2 Cat, TB; Liu-DeRyke, X (September 2010). "Medical management of variceal hemorrhage". Critical care nursing clinics of North America. 22 (3): 381–93. doi:10.1016/j.ccell.2010.02.004. PMID 20691388.
- ↑ "Octreotide Use During Pregnancy". Drugs.com. Archived from the original on 20 January 2021. Retrieved 25 December 2020.
- 1 2 3 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 1002. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ↑ MD, Laura Lamps; MD, Andrew Bellizzi; MD, Wendy L. Frankel; MD, Rhonda Yantiss; MD, Scott R. Owens (2015). Neoplastic Gastrointestinal Pathology: An Illustrated Guide. Springer Publishing Company. p. 40. ISBN 978-1-936287-72-7. Archived from the original on 28 August 2021. Retrieved 25 December 2020.
- ↑ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ↑ "Octreotide Monograph for Professionals". Drugs.com. Archived from the original on 24 January 2021. Retrieved 25 December 2020.
- ↑ "Octreotide Prices and Octreotide Coupons". GoodRx. Retrieved 25 December 2020.
- ↑ Octreotide Monograph
- ↑ Moattari AR, Cho K, Vinik AI (1990). "Somatostatin analogue in treatment of coexisting glucagonoma and pancreatic pseudocyst: dissociation of responses". Surgery. 108 (3): 581–7. PMID 2168587.
- ↑ Gøtzsche PC, Hróbjartsson A (July 2008). "Somatostatin analogues for acute bleeding oesophageal varices". The Cochrane Database of Systematic Reviews (3): CD000193. doi:10.1002/14651858.CD000193.pub3. PMC 7043291. PMID 18677774.
- ↑ "Medscape: Octreoscan review". Archived from the original on 12 February 2017. Retrieved 28 October 2010.
- ↑ Chin J, Vesnaver M, Bernard-Gauthier V, Saucke-Lacelle E, Wängler B, Wängler C, Schirrmacher R (November 2013). "Direct one-step labeling of cysteine residues on peptides with [(11)C]methyl triflate for the synthesis of PET radiopharmaceuticals". Amino Acids. 45 (5): 1097–108. doi:10.1007/s00726-013-1562-5. PMID 23921782.
- ↑ "Octreotide Capsules - Our Research". Chiasma. 24 January 2020. Archived from the original on 2 July 2020. Retrieved 30 June 2020.
- 1 2 "Chiasma Announces FDA Approval of Mycapssa (Octreotide) Capsules, the First and Only Oral Somatostatin Analog". Chiasma, Inc. (Press release). 26 June 2020. Archived from the original on 30 June 2020. Retrieved 30 June 2020.
- ↑ Antunes, C; Copelin II, EL (January 2021). "Upper Gastrointestinal Bleeding". PMID 29262121.
{{cite journal}}: Cite journal requires|journal=(help) - 1 2 3 4 5 Haberfeld, H, ed. (2009). Austria-Codex (in Deutsch) (2009/2010 ed.). Vienna: Österreichischer Apothekerverlag. ISBN 978-3-85200-196-8.
- 1 2 3 4 Dinnendahl, V; Fricke, U, eds. (2010). Arzneistoff-Profile (in Deutsch). Vol. 8 (23 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
- ↑ Hovind P, Simonsen L, Bülow J (March 2010). "Decreased leg glucose uptake during exercise contributes to the hyperglycaemic effect of octreotide". Clinical Physiology and Functional Imaging. 30 (2): 141–5. doi:10.1111/j.1475-097X.2009.00917.x. PMID 20132129.
- ↑ van der Lely AJ, de Herder WW, Lamberts SW (November 1997). "A risk-benefit assessment of octreotide in the treatment of acromegaly". Drug Safety. 17 (5): 317–24. doi:10.2165/00002018-199717050-00004. PMID 9391775.
- ↑ Kapicioglu S, Mollamehmetoglu M, Kutlu N, Can G, Ozgur GK (January 1998). "Inhibition of penile erection in rats by a long-acting somatostatin analogue, octreotide (SMS 201-995)". British Journal of Urology. 81 (1): 142–5. doi:10.1046/j.1464-410x.1998.00520.x. PMID 9467491.
- ↑ Klopp, T, ed. (2010). Arzneimittel-Interaktionen (in Deutsch) (2010/2011 ed.). Arbeitsgemeinschaft für Pharmazeutische Information. ISBN 978-3-85200-207-1.
- ↑ Maurer R, Gaehwiler BH, Buescher HH, Hill RC, Roemer D (August 1982). "Opiate antagonistic properties of an octapeptide somatostatin analog". Proceedings of the National Academy of Sciences of the United States of America. 79 (15): 4815–7. Bibcode:1982PNAS...79.4815M. doi:10.1073/pnas.79.15.4815. PMC 346769. PMID 6126877.
- ↑ Allen MP, Blake JF, Bryce DK, Haggan ME, Liras S, McLean S, Segelstein BE (March 2000). "Design, synthesis and biological evaluation of 3-amino-3-phenylpropionamide derivatives as novel mu opioid receptor ligands". Bioorganic & Medicinal Chemistry Letters. 10 (6): 523–6. doi:10.1016/s0960-894x(00)00034-2. PMID 10741545.
- ↑ "Sandostatin Lar Depot- octreotide acetate kit". DailyMed. 11 April 2019. Archived from the original on 24 March 2021. Retrieved 16 February 2020.
- 1 2 "Bynfezia Pen (octreotide acetate) injection, for subcutaneous use" (PDF). Sun Pharmaceutical Industries. 28 January 2020. Archived (PDF) from the original on 17 February 2020. Retrieved 16 February 2020.
- ↑ "Bynfezia Pen letter" (PDF). U.S. Food and Drug Administration (FDA). 28 January 2020. Archived (PDF) from the original on 17 February 2020. Retrieved 16 February 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ Matharu MS, Levy MJ, Meeran K, Goadsby PJ (October 2004). "Subcutaneous octreotide in cluster headache: randomized placebo-controlled double-blind crossover study". Annals of Neurology. 56 (4): 488–94. doi:10.1002/ana.20210. PMID 15455406.
- ↑ Uhl W, Anghelacopoulos SE, Friess H, Büchler MW (1999). "The role of octreotide and somatostatin in acute and chronic pancreatitis". Digestion. 60 Suppl 2 (2): 23–31. doi:10.1159/000051477. PMID 10207228.
- ↑ Shima Y, Ohtsu A, Shirao K, Sasaki Y (May 2008). "Clinical efficacy and safety of octreotide (SMS201-995) in terminally ill Japanese cancer patients with malignant bowel obstruction". Japanese Journal of Clinical Oncology. 38 (5): 354–9. doi:10.1093/jjco/hyn035. PMID 18490369.
- ↑ Skagen C, Einstein M, Lucey MR, Said A (August 2009). "Combination treatment with octreotide, midodrine, and albumin improves survival in patients with type 1 and type 2 hepatorenal syndrome". Journal of Clinical Gastroenterology. 43 (7): 680–5. doi:10.1097/MCG.0b013e318188947c. PMID 19238094.
- ↑ Patient.info (Feb 2013). "Hypotension". Archived from the original on 28 August 2021. Retrieved 26 June 2015.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Kilic D, Sahin E, Gulcan O, Bolat B, Turkoz R, Hatipoglu A (2005). "Octreotide for treating chylothorax after cardiac surgery". Texas Heart Institute Journal. 32 (3): 437–9. PMC 1336729. PMID 16392238.
- ↑ Siu SL, Lam DS (2006). "Spontaneous neonatal chylothorax treated with octreotide". Journal of Paediatrics and Child Health. 42 (1–2): 65–7. doi:10.1111/j.1440-1754.2006.00788.x. PMID 16487393.
- ↑ Chan EH, Russell JL, Williams WG, Van Arsdell GS, Coles JG, McCrindle BW (November 2005). "Postoperative chylothorax after cardiothoracic surgery in children". The Annals of Thoracic Surgery. 80 (5): 1864–70. doi:10.1016/j.athoracsur.2005.04.048. PMID 16242470.
- ↑ Greek Researchers Investigate Octreotide Archived 2010-12-19 at the Wayback Machine Hypertension Research Foundation, accessed 2011-01-02
- ↑ Panagopoulos GN, Deftereos SN, Tagaris GA, Gryllia M, Kounadi T, Karamani O, et al. (July 2007). "Octreotide: a therapeutic option for idiopathic intracranial hypertension". Neurology, Neurophysiology, and Neuroscience: 1. PMID 17700925.
- 1 2 3 4 Lustig RH, Hinds PS, Ringwald-Smith K, Christensen RK, Kaste SC, Schreiber RE, et al. (June 2003). "Octreotide therapy of pediatric hypothalamic obesity: a double-blind, placebo-controlled trial". The Journal of Clinical Endocrinology and Metabolism. 88 (6): 2586–92. doi:10.1210/jc.2002-030003. PMID 12788859.
- ↑ Flier JS (January 2004). "Obesity wars: molecular progress confronts an expanding epidemic". Cell. 116 (2): 337–50. doi:10.1016/S0092-8674(03)01081-X. PMID 14744442.
- ↑ Boulpaep, Emile L.; Boron, Walter F. (2003). Medical physiologya: A cellular and molecular approach. Philadelphia: Saunders. p. 1227. ISBN 978-0-7216-3256-8.
- ↑ Lustig RH (2011). "Hypothalamic obesity after craniopharyngioma: mechanisms, diagnosis, and treatment". Frontiers in Endocrinology. 2: 60. doi:10.3389/fendo.2011.00060. PMC 3356006. PMID 22654817.
- ↑ Lustig RH, Greenway F, Velasquez-Mieyer P, Heimburger D, Schumacher D, Smith D, et al. (February 2006). "A multicenter, randomized, double-blind, placebo-controlled, dose-finding trial of a long-acting formulation of octreotide in promoting weight loss in obese adults with insulin hypersecretion". International Journal of Obesity. 30 (2): 331–41. doi:10.1038/sj.ijo.0803074. PMC 1540404. PMID 16158082.
External links
| External sites: |
|
|---|---|
| Identifiers: |
|