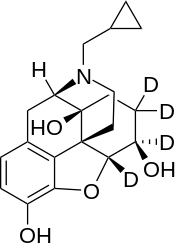
6β-Naltrexol-d4
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C20H21D4NO4 |
| Molar mass | 347.445 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
6β-Naltrexol-d4, also known as 6β-hydroxynaltrexone-d4, is a deuterium-labeled form of 6β-naltrexol used for NMR imaging. Unlike opioid inverse agonists such as naloxone and naltrexone (which are often dubbed "antagonists" for simplicity's sake), 6β-naltrexol and 6β-naltrexol-d4 are opioid neutral antagonists.[1]
References
- ↑ Sirohi S, Dighe SV, Madia PA, Yoburn BC (August 2009). "The relative potency of inverse opioid agonists and a neutral opioid antagonist in precipitated withdrawal and antagonism of analgesia and toxicity". The Journal of Pharmacology and Experimental Therapeutics. 330 (2): 513–9. doi:10.1124/jpet.109.152678. PMC 2713087. PMID 19435929.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.