Piritramide
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Dipidolor |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral, IM, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 95%[1] |
| Metabolism | Liver |
| Elimination half-life | 4-10 hours (acute dosing), 17.4 hours (chronic dosing) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.569 |
| Chemical and physical data | |
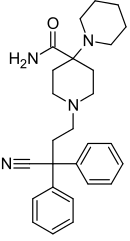
| Formula | C27H34N4O |
| Molar mass | 430.596 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Piritramide (R-3365, trade names Dipidolor, Piridolan, Pirium and others) is a synthetic opioid analgesic (narcotic painkiller) that is marketed in certain European countries including: Austria, Belgium, Czech Republic, Germany and the Netherlands.[2] It comes in free form, is about 0.75x times as potent as morphine and is given parenterally (by injection) for the treatment of severe pain.[2][3] Nausea, vomiting, respiratory depression and constipation are believed to be less frequent with piritramide than with morphine (the gold standard opioid against which other opioids are compared and contrasted), and it produces more rapid-onset analgesia (pain relief) when compared to morphine and pethidine. After intravenous administration the onset of analgesia is as little as 1–2 minutes, which may be related to its great lipophilicity.[4] The analgesic and sedative effects of piritramide are believed to be potentiated with phenothiazines and its emetic (nausea/vomiting-inducing) effects are suppressed.[4] The volume of distribution is 0.7-1 L/kg after a single dose, 4.7-6 L/kg after steady-state concentrations are achieved and up to 11.1 L/kg after prolonged dosing.[4]
Piritramide was developed and patented in Belgium, at Janssen, in 1960. It is part of an eponymous two-member class of opioids in clinical use with the other being bezitramide (Burgodin). The closest chemical and structural relatives of piritramide in clinical use include the diphenoxylate family, fentanyl (both Janssen discoveries) and somewhat more distantly alphaprodine.
Not being in clinical use in the United States, it is a Schedule I Narcotic controlled substance with a DEA ACSCN of 9642 and manufacturing quota of zero.[5]
See also
References
- ↑ Jage J, Laufenberg-Feldmann R, Heid F (May 2008). "[Drugs for postoperative analgesia: routine and new aspects: Part 2: opioids, ketamine and gabapentinoids]" [Drugs for postoperative analgesia: routine and new aspects: Part 2: opioids, ketamine and gabapentinoids]. Der Anaesthesist (in German). 57 (5): 491–8. doi:10.1007/s00101-008-1327-9. PMID 18409073.
- 1 2 Brayfield A, ed. (23 September 2011). "Piritramide". Martindale: The Complete Drug Reference. Pharmaceutical Press. Retrieved 22 April 2014.
- ↑ Kay B (December 1971). "A clinical investigation of piritramide in the treatment of postoperative pain". British Journal of Anaesthesia. 43 (12): 1167–71. doi:10.1093/bja/43.12.1167. PMID 4945251. S2CID 17729725.
- 1 2 3 "FACHINFORMATION (Zusammenfassung der Merkmale des Arzneimittels)" [PROFESSIONAL INFORMATION (Summary of Product Characteristics)] (PDF). Janssen. Janssen - Cilag Pharma GmbH. November 2013. Retrieved 9 April 2014.
- ↑ "DEA Diversion Control Division".