Morphiceptin
 | |
| Names | |
|---|---|
| IUPAC name
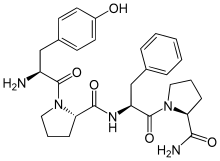
(2S)-1-[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]-N-[(2S)-1-[(2S)-2-carbamoylpyrrolidin-1-yl]-1-oxo-3-phenylpropan-2-yl]pyrrolidine-2-carboxamide[1] | |
| Other names
Tyr-Pro-Phe-Pro-NH2, PLO17 | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C28H35N5O5 |
| Molar mass | 521.6 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Morphiceptin is a tetrapeptide (Tyr-Pro-Phe-Pro-NH2) that is a selective μ-opioid receptor agonist. It is derived from β-casomorphin and has over 1,000 times selectivity for μ- over δ-opioid receptors. When injected intracerebroventricularly (into the ventricular system of the brain), morphiceptin had an analgesic ED50 of 1.7 nmol per animal. The analgesic effects of morphiceptin were reversed by naloxone, meaning that the analgesic effect is mediated by the μ-opioid receptor.[2]
Morphiceptin is the (1S,2S,3S,4S)-form whereas deproceptin is the (1S,2S,3S,4R)-form [84799-23-5].
See also
References
- ↑ "Morphiceptin". ChemBase. Archived from the original on 15 March 2012. Retrieved 1 August 2011.
- ↑ Chang, K (3 May 1982). "Analgesic activity of intracerebroventricular administration of morphiceptin and β-casomorphins: Correlation with the morphine (μ) receptor binding affinity". Life Sciences. 30 (18): 1547–1551. doi:10.1016/0024-3205(82)90242-9. PMID 6281604.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.