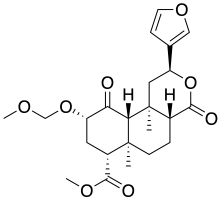
Salvinorin B methoxymethyl ether
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C23H30O8 |
| Molar mass | 434.485 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Salvinorin B methoxymethyl ether (2-O-methoxymethylsalvinorin B) is a semi-synthetic analogue of the natural product salvinorin A used in scientific research.[1][2] It has a longer duration of action of around 2–3 hours, compared to less than 30 minutes for salvinorin A,[3] and has increased affinity and potency at the κ-opioid receptor. It is made from salvinorin B, which is most conveniently made from salvinorin A by deacetylation.[4] The crystal structure reveals that the methoxy group overlaps with the acetyl group of salvinorin A, but with a different orientation.[5]
Salvinorin B methoxymethyl ether has a Ki of 0.60 nM at the κ opioid receptor,[6] and is around five times more potent than salvinorin A in animal studies, although it is still only half as potent as its stronger homolog salvinorin B ethoxymethyl ether (symmetry).[7]
See also
References
- ↑ Inan S, Lee DY, Liu-Chen LY, Cowan A (March 2009). "Comparison of the diuretic effects of chemically diverse kappa opioid agonists in rats: nalfurafine, U50,488H, and salvinorin A". Naunyn-Schmiedeberg's Archives of Pharmacology. 379 (3): 263–70. doi:10.1007/s00210-008-0358-8. PMID 18925386. S2CID 8123431.
- ↑ McLennan GP, Kiss A, Miyatake M, Belcheva MM, Chambers KT, Pozek JJ, et al. (December 2008). "Kappa opioids promote the proliferation of astrocytes via Gbetagamma and beta-arrestin 2-dependent MAPK-mediated pathways". Journal of Neurochemistry. 107 (6): 1753–65. doi:10.1111/j.1471-4159.2008.05745.x. PMC 2606093. PMID 19014370.
- ↑ Wang Y, Chen Y, Xu W, Lee DY, Ma Z, Rawls SM, et al. (March 2008). "2-Methoxymethyl-salvinorin B is a potent kappa opioid receptor agonist with longer lasting action in vivo than salvinorin A". The Journal of Pharmacology and Experimental Therapeutics. 324 (3): 1073–83. doi:10.1124/jpet.107.132142. PMC 2519046. PMID 18089845.
- ↑ Lee DY, Karnati VV, He M, Liu-Chen LY, Kondaveti L, Ma Z, et al. (August 2005). "Synthesis and in vitro pharmacological studies of new C(2) modified salvinorin A analogues". Bioorganic & Medicinal Chemistry Letters. 15 (16): 3744–7. doi:10.1016/j.bmcl.2005.05.048. PMID 15993589.
- ↑ Munro TA, Ho DM, Cohen BM (November 2012). "Salvinorin B methoxymethyl ether". Acta Crystallographica Section E. 68 (Pt 11): o3225-6. doi:10.1107/s1600536812043449. PMC 3515309. PMID 23284529.
- ↑ Munro TA, Duncan KK, Xu W, Wang Y, Liu-Chen LY, Carlezon WA, et al. (February 2008). "Standard protecting groups create potent and selective kappa opioids: salvinorin B alkoxymethyl ethers". Bioorganic & Medicinal Chemistry. 16 (3): 1279–86. doi:10.1016/j.bmc.2007.10.067. PMC 2568987. PMID 17981041.
- ↑ Baker LE, Panos JJ, Killinger BA, Peet MM, Bell LM, Haliw LA, Walker SL (April 2009). "Comparison of the discriminative stimulus effects of salvinorin A and its derivatives to U69,593 and U50,488 in rats". Psychopharmacology. 203 (2): 203–11. doi:10.1007/s00213-008-1458-3. PMID 19153716.