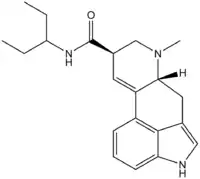
Lysergic acid 3-pentyl amide
 | |
| Clinical data | |
|---|---|
| Other names | Lysergic acid 3-pentyl amide |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H27N3O |
| Molar mass | 337.467 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Lysergic acid 3-pentyl amide (3-Pentyllysergamide, LSP) is an analogue of LSD originally researched by David E. Nichols and colleagues at Purdue University. It has similar binding affinity to LSD itself as both a 5-HT1A and 5-HT2A agonist, and produces similar behavioral and physiological responses in animals with only slightly lower potency than LSD. Other isomers of this compound have also been explored, with the 1-pentylamide being around 75% the potency of LSD,[1] while the (R)-2-pentylamide shows similar 5-HT2A binding affinity to LSD in vitro but has only around half the potency of LSD in producing drug-appropriate responding in mice, and the (S)-2-pentylamide is inactive.[2]
See also
References
- ↑ Nichols DE (2001). "LSD and Its Lysergamide Cousins". The Heffter Review of Psychedelic Research. 2: 80–87.
- ↑ Monte AP, Marona-Lewicka D, Kanthasamy A, Sanders-Bush E, Nichols DE (March 1995). "Stereoselective LSD-like activity in a series of d-lysergic acid amides of (R)- and (S)-2-aminoalkanes". Journal of Medicinal Chemistry. 38 (6): 958–66. doi:10.1021/jm00006a015. PMID 7699712.
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Lysergic acid derivatives |
|
|---|---|
| Psychedelic lysergamides |
|
| Clavines |
|
| Other ergolines |
|
| Natural sources |
Morning glory: Argyreia nervosa (Hawaiian Baby Woodrose), Ipomoea spp.(Morning Glory, Tlitliltzin, Badoh Negro), Rivea corymbosa (Coaxihuitl, Ololiúqui) |