AR-A000002
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
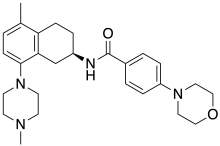
| Formula | C27H36N4O2 |
| Molar mass | 448.611 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
AR-A000002 is a drug which is one of the first compounds developed to act as a selective antagonist for the serotonin receptor 5-HT1B,[1] with approximately 10x selectivity for 5-HT1B over the closely related 5-HT1D receptor.[2] It has been shown to produce sustained increases in levels of serotonin in the brain, and has anxiolytic effects in animal studies.[3][4]
References
- ↑ Hudzik TJ, Yanek M, Porrey T, Evenden J, Paronis C, Mastrangelo M, et al. (March 2003). "Behavioral pharmacology of AR-A000002, a novel, selective 5-hydroxytryptamine(1B) antagonist". The Journal of Pharmacology and Experimental Therapeutics. 304 (3): 1072–84. doi:10.1124/jpet.102.045468. PMID 12604684. S2CID 20463714.
- ↑ Ahlgren C, Eriksson A, Tellefors P, Ross SB, Stenfors C, Malmberg A (September 2004). "In vitro characterization of AR-A000002, a novel 5-hydroxytryptamine(1B) autoreceptor antagonist". European Journal of Pharmacology. 499 (1–2): 67–75. doi:10.1016/j.ejphar.2004.07.067. PMID 15363952.
- ↑ Stenfors C, Ahlgren C, Yu H, Wedén M, Larsson LG, Ross SB (March 2004). "Effects of long-term administration of the 5-hydroxytryptamine1B receptor antagonist AR-A000002 to guinea pigs". Psychopharmacology. 172 (3): 333–40. doi:10.1007/s00213-003-1667-8. PMID 14652708. S2CID 25130658.
- ↑ Stenfors C, Hallerbäck T, Larsson LG, Wallsten C, Ross SB (March 2004). "Pharmacology of a novel selective 5-hydroxytryptamine1B receptor antagonist, AR-A000002". Naunyn-Schmiedeberg's Archives of Pharmacology. 369 (3): 330–7. doi:10.1007/s00210-004-0866-0. PMID 14758468. S2CID 21075322.
| 5-HT1AR agonists | |
|---|---|
| GABAAR PAMs |
|
| Gabapentinoids (α2δ VDCC blockers) | |
| Antidepressants |
|
| Sympatholytics (Antiadrenergics) |
|
| Others | |
| |
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.