SB-216641
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
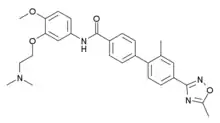
| Formula | C28H30N4O4 |
| Molar mass | 486.572 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
SB-216641 is a drug which is a selective antagonist for the serotonin receptor 5-HT1B, with around 25x selectivity over the closely related 5-HT1D receptor.[1] It is used in scientific research,[2][3][4] and has demonstrated anxiolytic effects in animal studies.[5][6]
References
- ↑ Price GW, Burton MJ, Collin LJ, Duckworth M, Gaster L, Göthert M, et al. (September 1997). "SB-216641 and BRL-15572--compounds to pharmacologically discriminate h5-HT1B and h5-HT1D receptors". Naunyn-Schmiedeberg's Archives of Pharmacology. 356 (3): 312–20. doi:10.1007/PL00005056. PMID 9303567. S2CID 26760453.
- ↑ Matsuoka T, Hasuo H, Akasu T (March 2004). "5-Hydroxytryptamine 1B receptors mediate presynaptic inhibition of monosynaptic IPSC in the rat dorsolateral septal nucleus". Neuroscience Research. 48 (3): 229–38. doi:10.1016/j.neures.2003.11.004. PMID 15154669. S2CID 35850798.
- ↑ Yan QS, Zheng SZ, Yan SE (September 2004). "Involvement of 5-HT1B receptors within the ventral tegmental area in regulation of mesolimbic dopaminergic neuronal activity via GABA mechanisms: a study with dual-probe microdialysis". Brain Research. 1021 (1): 82–91. doi:10.1016/j.brainres.2004.06.053. PMID 15328035. S2CID 46700479.
- ↑ Lee JJ, Hahm ET, Lee CH, Cho YW (January 2008). "Serotonergic modulation of GABAergic and glutamatergic synaptic transmission in mechanically isolated rat medial preoptic area neurons". Neuropsychopharmacology. 33 (2): 340–52. doi:10.1038/sj.npp.1301396. PMID 17392733.
- ↑ Tatarczyńska E, Kłodzińska A, Stachowicz K, Chojnacka-Wójcik E (December 2004). "Effects of a selective 5-HT1B receptor agonist and antagonists in animal models of anxiety and depression". Behavioural Pharmacology. 15 (8): 523–34. doi:10.1097/00008877-200412000-00001. PMID 15577451. S2CID 6940756.
- ↑ Chojnacka-Wójcik E, Kłodzińska A, Tatarczyńska E (February 2005). "The anxiolytic-like effect of 5-HT1B receptor ligands in rats: a possible mechanism of action". The Journal of Pharmacy and Pharmacology. 57 (2): 253–7. doi:10.1211/0022357055399. PMID 15720791. S2CID 20743875.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.