Psilocybin
 | |
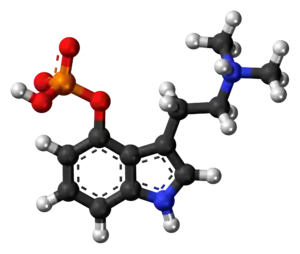 Kekulé, skeletal formula of canonical psilocybin (top) and spacefill model (bottom) | |
| Names | |
|---|---|
| Pronunciation | /ˌsaɪləˈsaɪbɪn/ sy-lə-SY-bin |
| Other names | 4-PO-DMT, psilocybine, psilocibin, psilocybinum, psilotsibin, psilocin phosphate ester, indocybin[1] |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Dependence risk | Low |
| Routes of use | By mouth, intravenous |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Metabolism | Liver |
| Excretion | Kidney |
| Chemical and physical data | |
| Formula | C12H17N2O4P |
| Molar mass | 284.252 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 220–228 °C (428–442 °F) |
| Solubility in water | Soluble in boiling methanol and water, slightly soluble in ethanol, insoluble in chloroform, benzene[2] |
SMILES
| |
InChI
| |
Psilocybin is a naturally occurring psychedelic. In general, the effects include euphoria, visual and mental hallucinations, changes in perception, a distorted sense of time, and perceived spiritual experiences. Side effects may include nausea and panic attacks. The intensity and duration of the effects of psilocybin are variable, depending on the type of mushrooms, dosage, individual, and set and setting. The mind-altering effects of psilocybin typically last from two to six hours.
It is produced by more than 200 species of fungi. The most potent are members of the Psilocybe, such as P. azurescens, P. semilanceata, and P. cyanescens, but psilocybin has also been isolated from about a dozen other types. Psilocybin is itself biologically inactive but is converted by the body to psilocin, which has mind-altering effects similar, in some aspects, to LSD, mescaline, and DMT. Once ingested, psilocybin is rapidly metabolized to psilocin, which then acts on serotonin receptors in the brain.
Imagery found on prehistoric murals and rock paintings suggests that human use of psilocybin mushrooms predates recorded history. In Mesoamerica, the mushrooms had long been consumed in spiritual and divinatory ceremonies before Spanish chroniclers first documented their use in the sixteenth century. In 1959, the Swiss chemist Albert Hofmann isolated the active principle psilocybin from the mushroom Psilocybe mexicana. Hofmann's employer Sandoz marketed and sold pure psilocybin to physicians and clinicians worldwide for use in psychedelic psychotherapy. Although the increasingly restrictive drug laws of the late 1960s curbed scientific research into the effects of psilocybin and other hallucinogens, its popularity as an entheogen (spirituality-enhancing agent) grew in the next decade, owing largely to the increased availability of information on how to cultivate psilocybin mushrooms. Possession has been outlawed in most countries, and it is classified as a scheduled drug by many nations.
Effects

Psilocybin may result in a wide range of effects including: feelings of disorientation, lethargy, giddiness, euphoria, joy, and depression. 31% of volunteers given a high dose report feeling fear and 17% experienced brief paranoia.[3] With a moderate dose, negative experiences are rare, whereas a third of those given a high dose experienced anxiety or paranoia.[4][5] Low doses can induce hallucinatory effects. Closed-eye hallucinations may occur, where the individual sees multicolored geometric shapes and vivid imaginative sequences.[6] Some individuals report synesthesia, such as tactile sensations when viewing colors.[7]: 175 At higher doses, psilocybin can lead to "intensification of affective responses, enhanced ability for introspection, regression to primitive and childlike thinking, and activation of vivid memory traces with pronounced emotional undertones".[8] Open-eye visual hallucinations are common, and may be very detailed although rarely confused with reality.[6]
The effects depend on the mindset and environment in which the user has the experience, factors commonly referred to as set and setting. In the early 1960s, Timothy Leary administered the drug to volunteers in an environment similar to a comfortable living room. Individuals who had experience with psilocybin prior reported more pleasant experiences than those for whom the drug was novel. Group size, dosage, preparation, and expectancy affected the response. It has been proposed that psilocybin heightens suggestibility, making an individual more receptive to interpersonal interactions and environmental stimuli.[9][10]
A single high dosage of psilocybin can cause long-term changes in personality. About half of the study participants—described as healthy, "spiritually active", and many possessing postgraduate degrees—showed an increase in the personality dimension of openness, and this effect was apparent more than a year after. According to the study authors, the finding is significant because "no study has prospectively demonstrated personality change in healthy adults after an experimentally manipulated discrete event."[11] A further study by Griffiths in 2017 found that doses of 20 to 30 mg/70 kg psilocybin inducing mystical-type experiences brought more lasting changes to traits including altruism, gratitude, forgiveness and feeling close to others when they were combined with a regular meditation practice and an extensive spiritual practice support programme.[12][13] Although other researchers have described instances of psychedelic drug usage leading to new psychological understandings and personal insights,[14] it is not known whether these experimental results can be generalized to larger populations.[11]
Physical
Common responses include pupil dilation (93%); changes in heart rate (100%), including increases (56%), decreases (13%), and variable responses (31%); changes in blood pressure (84%), including hypotension (34%), hypertension (28%), and general instability (22%); changes in stretch reflex (86%), including increases (80%) and decreases (6%); nausea (44%); tremor (25%); and dysmetria (16%) (inability to properly direct or limit motions).[lower-alpha 1] The temporary increases in blood pressure caused by the drug can be a risk factor for users with pre-existing hypertension.[6] These qualitative somatic effects caused by psilocybin have been corroborated by several early clinical studies.[17] A 2005 magazine survey of club goers in the UK found that nausea or vomiting was experienced by over a quarter of those who had used psilocybin mushrooms in the last year, although this effect is caused by the mushroom rather than psilocybin itself.[3] In one study, administration of gradually increasing dosages of psilocybin daily for 21 days had no measurable effect on electrolyte levels, blood sugar levels, or liver toxicity tests.[15]
Perceptual
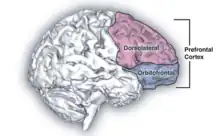
Psilocybin is known to strongly influence the subjective experience of the passage of time.[18] Users often feel as if time is slowed down, resulting in the perception that "minutes appear to be hours" or "time is standing still".[19] Studies have demonstrated that psilocybin significantly impairs subjects' ability to gauge time intervals longer than 2.5 seconds, impairs their ability to synchronize to inter-beat intervals longer than 2 seconds, and reduces their preferred tapping rate.[19][20] These results are consistent with the drug's role in affecting prefrontal cortex activity,[21] and the role that the prefrontal cortex is known to play in time perception.[22] However, the neurochemical basis of psilocybin's effects on the perception of time are not known with certainty.[23]
Users having a pleasant experience can feel a sense of connection to others, nature, and the universe; other perceptions and emotions are also often intensified. Users having an unpleasant experience (a "bad trip") describe a reaction accompanied by fear, other unpleasant feelings, and occasionally by dangerous behavior. In general, the phrase "bad trip" is used to describe a reaction that is characterized primarily by fear or other unpleasant emotions, not just transitory experience of such feelings. A variety of factors may contribute to a psilocybin user experiencing a bad trip, including "tripping" during an emotional or physical low or in a non-supportive environment (see: set and setting). Ingesting psilocybin in combination with other drugs, including alcohol, can also increase the likelihood of a bad trip.[3][24] Other than the duration of the experience, the effects of psilocybin are similar to comparable dosages of LSD or mescaline. However, in the Psychedelics Encyclopedia, author Peter Stafford noted, "The psilocybin experience seems to be warmer, not as forceful and less isolating. It tends to build connections between people, who are generally much more in communication than when they use LSD."[25]: 273
Groups
In many traditional societies that mind-active substances such as psilocybin are regularly "consumed ritually for therapeutic purposes or for transcending normal, everyday reality."[26][27] Positive effects that psilocybin has on individuals can be observed by taking on an anthropological approach and moving away from the Western bio-medical society; this is aided by the studies done by Leary.[28] Within certain traditional societies where the use of psilocybin is frequent for shamanic healing rituals, group collectives praise their guide, healer and shaman for helping alleviate them of pains, aches and hurt. They do this through a group ritual practice where participants, or just the guide, ingests psilocybin to help extract any "toxic psychic residues or sorcerous implants"[27][29] found in one's body. Group therapies using classic psychedelics are becoming more commonly used in the Western world in clinical practice. This may continue to grow as long providing the evidence remains indicative of safety and efficacy.[30] In social sense, the group is shaped by their experiences surrounding psilocybin and how they view the plant collectively. As mentioned in anthropologist article,[27] the group partake in a "journey" together, thus adding to the spiritual, social body, where roles, hierarchies and gender are subjectively understood.[27]
Mystical
Psilocybin mushrooms have been and continue to be used in indigenous New World cultures in religious, divinatory, or spiritual contexts. Reflecting the meaning of the word entheogen ("the god within"), the mushrooms are revered as powerful spiritual sacraments that provide access to sacred worlds. Typically used in small group community settings, they enhance group cohesion and reaffirm traditional values.[31] Terence McKenna documented the worldwide practices of psilocybin mushroom usage as part of a cultural ethos relating to the Earth and mysteries of nature, and suggested that mushrooms enhanced self-awareness and a sense of contact with a "Transcendent Other"—reflecting a deeper understanding of our connectedness with nature.[32]
Psychedelic drugs can induce states of consciousness that have lasting personal meaning and spiritual significance in individuals who are religious or spiritually inclined; these states are called mystical experiences. Some scholars have proposed that many of the qualities of a drug-induced mystical experience are indistinguishable from mystical experiences achieved through non-drug techniques, such as meditation or holotropic breathwork.[33][34] In the 1960s, Walter Pahnke and colleagues systematically evaluated mystical experiences (which they called "mystical consciousness") by categorizing their common features. These categories, according to Pahnke, "describe the core of a universal psychological experience, free from culturally determined philosophical or theological interpretations", and allow researchers to assess mystical experiences on a qualitative, numerical scale.[35]
In the 1962 Marsh Chapel Experiment,[36] almost all of the graduate degree divinity student volunteers who received psilocybin reported religious experiences.[37] One of the participants was religious scholar Huston Smith; he later described his experience as "the most powerful cosmic homecoming I have ever experienced."[38] In a 25-year followup to the experiment, all of the subjects given psilocybin described their experience as having elements of "a genuine mystical nature and characterized it as one of the high points of their spiritual life".[39]: 13 Psychedelic researcher Rick Doblin considered the study partially flawed due to incorrect implementation of the double-blind procedure, and several imprecise questions in the mystical experience questionnaire. Nevertheless, he said that the study cast "a considerable doubt on the assertion that mystical experiences catalyzed by drugs are in any way inferior to non-drug mystical experiences in both their immediate content and long-term effects".[39]: 24 This sentiment was echoed by psychiatrist William A. Richards, who in a 2007 review stated "[psychedelic] mushroom use may constitute one technology for evoking revelatory experiences that are similar, if not identical, to those that occur through so-called spontaneous alterations of brain chemistry."[40]
After 14 months, those who reported mystical experiences scored on average 4 percentage points higher on the personality trait of Openness/Intellect; personality traits are normally stable across the lifespan for adults. Likewise, in a recent (2010) web-based questionnaire study designed to investigate user perceptions of the benefits and harms of hallucinogenic drug use, 60% of the 503 psilocybin users reported that their use of psilocybin had a long-term positive impact on their sense of well-being.[3][41]
While many recent studies have concluded that psilocybin can cause mystical-type experiences having substantial and sustained personal meaning and spiritual significance, not all the medical community agree. Paul R. McHugh, responded as follows in a book review: "The unmentioned fact in The Harvard Psychedelic Club is that LSD, psilocybin, mescaline, and the like produce not a "higher consciousness" but rather a particular kind of "lower consciousness" known well to psychiatrists and neurologists—namely,toxic delirium."[42]
Available forms
Although psilocybin may be prepared synthetically, outside of the research setting it is not typically used in this form. The psilocybin present in certain species of mushrooms can be ingested in several ways: by consuming fresh or dried fruit bodies, by preparing an herbal tea, or by combining with other foods to mask the bitter taste.[43] In rare cases people have injected mushroom extracts intravenously.[3]
Side effects
Most of the comparatively few fatal incidents reported in the literature that are associated with psychedelic mushroom usage involve the simultaneous use of other drugs, especially alcohol. Probably the most common cause of hospital admissions resulting from psychedelic mushroom usage involve "bad trips" or panic reactions, in which affected individuals become extremely anxious, confused, agitated, or disoriented. Accidents, self-injury, or suicide attempts can result from serious cases of acute psychotic episodes.[3] Although no studies have linked psilocybin with birth defects,[44] it is recommended that pregnant women avoid its usage.[45]
Toxicity

Data is sparse, but in the decade leading up to 2020 an increasing number of psilocybin mushroom overdoses have been recorded. One analysis of mushrooms used by people hospitalized from psilocybin poisoning found high concentrations of phenethylamine (PEA), which has also been detected in the urine of people who have used psilocybin mushrooms. It is hypothesized that PEA may intensify the effect of psilocybin poisoning.[47]
In rats, the median lethal dose (LD50) when administered orally is 280 milligrams per kilogram (mg/kg), approximately one and a half times that of caffeine. When administered intravenously in rabbits, psilocybin's LD50 is approximately 12.5 mg/kg.[48] Psilocybin comprises approximately 1% of the weight of Psilocybe cubensis mushrooms, and so nearly 1.7 kilograms (3.7 lb) of dried mushrooms, or 17 kilograms (37 lb) of fresh mushrooms, would be required for a 60-kilogram (130 lb) person to reach the 280 mg/kg LD50 value of rats.[3] Based on the results of animal studies, the lethal dose of psilocybin has been extrapolated to be 6 grams, 1000 times greater than the effective dose of 6 milligrams.[49] The Registry of Toxic Effects of Chemical Substances assigns psilocybin a relatively high therapeutic index of 641 (higher values correspond to a better safety profile); for comparison, the therapeutic indices of aspirin and nicotine are 199 and 21, respectively.[50] The lethal dose from psilocybin toxicity alone is unknown at recreational or medicinal levels, and has rarely been documented—as of 2011, only two cases attributed to overdosing on hallucinogenic mushrooms (without concurrent use of other drugs) have been reported in the scientific literature and may involve other factors aside from psilocybin.[3][lower-alpha 2]
Psychiatric
Panic reactions can occur after consumption of psilocybin-containing mushrooms, especially if the ingestion is accidental or otherwise unexpected. Reactions characterized by violent behavior, suicidal thoughts,[53] schizophrenia-like psychosis,[54][55] and convulsions[56] have been reported in the literature. A 2005 survey conducted in the United Kingdom found that almost a quarter of those who had used psilocybin mushrooms in the past year had experienced a panic attack.[3] Other adverse effects less frequently reported include paranoia, confusion, prolonged derealization (disconnection from reality), and mania.[41] Psilocybin usage can temporarily induce a state of depersonalization disorder.[57] Usage by those with schizophrenia can induce acute psychotic states requiring hospitalization.[58]
The similarity of psilocybin-induced symptoms to those of schizophrenia has made the drug a useful research tool in behavioral and neuroimaging studies of this psychotic disorder.[59][60][61] In both cases, psychotic symptoms are thought to arise from a "deficient gating of sensory and cognitive information" in the brain that ultimately lead to "cognitive fragmentation and psychosis".[60] Flashbacks (spontaneous recurrences of a previous psilocybin experience) can occur long after having used psilocybin mushrooms. Hallucinogen persisting perception disorder (HPPD) is characterized by a continual presence of visual disturbances similar to those generated by psychedelic substances. Neither flashbacks nor HPPD are commonly associated with psilocybin usage,[3] and correlations between HPPD and psychedelics are further obscured by polydrug use and other variables.[62]
Tolerance and dependence
Tolerance to psilocybin builds and dissipates quickly; ingesting psilocybin more than about once a week can lead to diminished effects. Tolerance dissipates after a few days, so doses can be spaced several days apart to avoid the effect.[63] A cross-tolerance can develop between psilocybin and the pharmacologically similar LSD,[64] and between psilocybin and phenethylamines such as mescaline and DOM.[65]
Repeated use of psilocybin does not lead to physical dependence.[15] A 2008 study concluded that, based on US data from the period 2000–2002, adolescent-onset (defined here as ages 11–17) usage of hallucinogenic drugs (including psilocybin) did not increase the risk of drug dependence in adulthood; this was in contrast to adolescent usage of cannabis, cocaine, inhalants, anxiolytic medicines, and stimulants, all of which were associated with "an excess risk of developing clinical features associated with drug dependence".[66] Likewise, a 2010 Dutch study ranked the relative harm of psilocybin mushrooms compared to a selection of 19 recreational drugs, including alcohol, cannabis, cocaine, ecstasy, heroin, and tobacco. Psilocybin mushrooms were ranked as the illicit drug with the lowest harm,[67] corroborating conclusions reached earlier by expert groups in the United Kingdom.[68]
Pharmacology
Pharmacodynamics
Psilocybin is rapidly dephosphorylated in the body to psilocin, which is an agonist for several serotonin receptors, which are also known as 5-hydroxytryptamine (5-HT) receptors. In rats, psilocin binds with high affinity to 5-HT2A receptors and low affinity to 5-HT1 receptors, including 5-HT1A and 5-HT1D; effects are also mediated via 5-HT2C receptors.[15] The psychotomimetic (psychosis-mimicking) effects of psilocin can be blocked in a dose-dependent fashion by the 5-HT2A antagonist drug ketanserin.[54] Various lines of evidence have shown that interactions with non-5-HT2 receptors also contribute to the subjective and behavioral effects of the drug.[65][lower-alpha 3] For example, psilocin indirectly increases the concentration of the neurotransmitter dopamine in the basal ganglia, and some psychotomimetic symptoms of psilocin are reduced by haloperidol, a non-selective dopamine receptor antagonist. Taken together, these suggest that there may be an indirect dopaminergic contribution to psilocin's psychotomimetic effects.[23] Psilocybin and psilocin have no affinity for dopamine receptor D2, unlike another common 5-HT receptor agonist, LSD.[15] Psilocin antagonizes H1 receptors with moderate affinity, compared to LSD which has a lower affinity.[70] Serotonin receptors are located in numerous parts of the brain, including the cerebral cortex, and are involved in a wide range of functions, including regulation of mood, motivation, body temperature, appetite and sex.[71]
Psilocybin induces region-dependent alterations in glutamate that may be associated with subjective experiences of ego dissolution.[72]
Pharmacokinetics
The effects of the drug begin 10–40 minutes after ingestion, and last 2–6 hours depending on dose, species, and individual metabolism.[73]: 36–41 The half life of psilocybin is 163 ± 64 minutes when taken orally, or 74.1 ± 19.6 minutes when injected intravenously.[15]
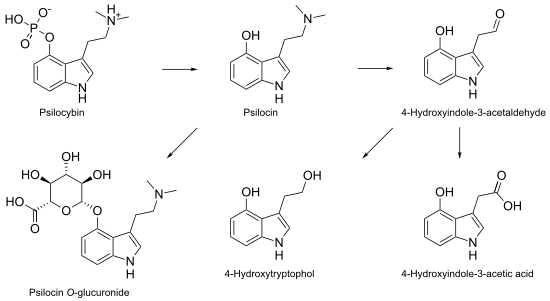
Psilocybin is metabolized mostly in the liver. As it becomes converted to psilocin, it undergoes a first-pass effect, whereby its concentration is greatly reduced before it reaches the systemic circulation. Psilocin is broken down by the enzyme monoamine oxidase to produce several metabolites that can circulate in the blood plasma, including 4-hydroxyindole-3-acetaldehyde, 4-hydroxytryptophol, and 4-hydroxyindole-3-acetic acid.[15] Some psilocin is not broken down by enzymes and instead forms a glucuronide; this is a biochemical mechanism animals use to eliminate toxic substances by linking them with glucuronic acid, which can then be excreted in the urine.[74][75] Psilocin is glucuronated by the glucuronosyltransferase enzymes UGT1A9 in the liver, and by UGT1A10 in the small intestine.[76] Based on studies using animals, about 50% of ingested psilocybin is absorbed through the stomach and intestine. Within 24 hours, about 65% of the absorbed psilocybin is excreted into the urine, and a further 15–20% is excreted in the bile and feces. Although most of the remaining drug is eliminated in this way within 8 hours, it is still detectable in the urine after 7 days.[77] Clinical studies show that psilocin concentrations in the plasma of adults average about 8 µg/liter within 2 hours after ingestion of a single 15 mg oral psilocybin dose;[78] psychological effects occur with a blood plasma concentration of 4–6 µg/liter.[15] Psilocybin is about 100 times less potent than LSD on a weight per weight basis, and the physiological effects last about half as long.[7]: 171
Monoamine oxidase inhibitors (MAOI) have been known to prolong and enhance the effects of DMT and one study assumed that the effect on psilocybin would be similar since it is a structural analogue of DMT.[79] Alcohol consumption may enhance the effects of psilocybin, because acetaldehyde, one of the primary breakdown metabolites of consumed alcohol, reacts with biogenic amines present in the body to produce MAOIs related to tetrahydroisoquinoline and β-carboline.[3] Tobacco smokers may also experience more powerful effects with psilocybin,[3] because tobacco smoke exposure decreases the activity of MAO in the brain and peripheral organs.[80]
Chemistry
Psilocybin (O-phosphoryl-4-hydroxy-N,N-dimethyltryptamine, 4-PO-Psilocin, or 4-PO-HO-DMT) is a prodrug that is converted into the pharmacologically active compound psilocin in the body by a dephosphorylation reaction. This chemical reaction takes place under strongly acidic conditions, or under physiological conditions in the body, through the action of enzymes called alkaline phosphatases.[81]
Psilocybin is a tryptamine compound with a chemical structure containing an indole ring linked to an ethylamine substituent. It is chemically related to the amino acid tryptophan, and is structurally similar to the neurotransmitter serotonin. Psilocybin is a member of the general class of tryptophan-based compounds that originally functioned as antioxidants in earlier life forms before assuming more complex functions in multicellular organisms, including humans.[82] Other related indole-containing psychedelic compounds include dimethyltryptamine, found in many plant species and in trace amounts in some mammals, and bufotenine, found in the skin of psychoactive toads.[83]: 10–13
Psilocybin is an alkaloid that is soluble in water, methanol and aqueous ethanol, but insoluble in organic solvents like chloroform and petroleum ether.[83]: 15 Its pKa values are estimated to be 1.3 and 6.5 for the two successive phosphate OH groups and 10.4 for the dimethylamine nitrogen, so in general it exists as a zwitterionic structure.[84] Exposure to light is detrimental to the stability of aqueous solutions of psilocybin, and will cause it to rapidly oxidize—an important consideration when using it as an analytical standard.[85] Osamu Shirota and colleagues reported a method for the large-scale synthesis of psilocybin without chromatographic purification in 2003.[86] Starting with 4-hydroxyindole, they generated psilocybin from psilocin in 85% yield, a marked improvement over yields reported from previous syntheses.[87][88][89] Purified psilocybin is a white, crystalline powder.[86] There are two known crystalline polymorphs of psilocybin, as well as reported hydrated phases.[90] The compound is reported to have a melting point between 220–228 °C (428–442 °F),[48] and a slightly ammonia-like taste.[84] In 2020, a second-generation synthesis of psilocybin has been developed.[91]
Biosynthetically, the biochemical transformation from tryptophan to psilocybin involves several enzyme reactions: decarboxylation, methylation at the N9 position, 4-hydroxylation, and O-phosphorylation. Isotopic labeling experiments from the 1960s suggested that tryptophan decarboxylation is the initial biosynthetic step and that O-phosphorylation is the final step,[92][93] but recent analyses of isolated enzymes demonstrate that O-phosphorylation is the third step in P. cubensis.[94] The sequence of the intermediate enzymatic steps has been shown to involve 4 different enzymes (PsiD, PsiH, PsiK, and PsiM) in P. cubensis and P. cyanescens, although the biosynthetic pathway may differ between species.[83]: 12–13 [94] These enzymes are encoded in gene clusters in Psilocybe, Panaeolus, and Gymnopilus.[95]
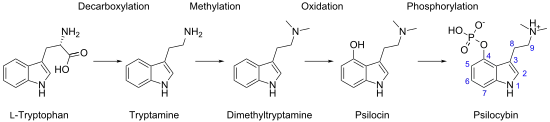
Researchers have genetically engineered Escherichia coli that can manufacture large amounts of psilocybin.[96] Psilocybin can be produced de novo in yeast.[97][98]
Analytical methods
Several relatively simple chemical tests—commercially available as reagent testing kits—can be used to assess the presence of psilocybin in extracts prepared from mushrooms. The drug reacts in the Marquis test to produce a yellow color, and a green color in the Mandelin reagent.[99] Neither of these tests, however, is specific for psilocybin; for example, the Marquis test will react with many classes of controlled drugs, such as those containing primary amino groups and unsubstituted benzene rings, including amphetamine and methamphetamine.[100] Ehrlich's reagent and DMACA reagent are used as chemical sprays to detect the drug after thin layer chromatography.[101] Many modern techniques of analytical chemistry have been used to quantify psilocybin levels in mushroom samples. Although the earliest methods commonly used gas chromatography, the high temperature required to vaporize the psilocybin sample prior to analysis causes it to spontaneously lose its phosphoryl group and become psilocin—making it difficult to chemically discriminate between the two drugs. In forensic toxicology, techniques involving gas chromatography coupled to mass spectrometry (GC–MS) are the most widely used due to their high sensitivity and ability to separate compounds in complex biological mixtures.[102] These techniques include ion mobility spectrometry,[103] capillary zone electrophoresis,[104] ultraviolet spectroscopy,[105] and infrared spectroscopy.[106] High-performance liquid chromatography (HPLC) is used with ultraviolet,[85] fluorescence,[107] electrochemical,[108] and electrospray mass spectrometric detection methods.[109]
Various chromatographic methods have been developed to detect psilocin in body fluids: the rapid emergency drug identification system (REMEDi HS), a drug screening method based on HPLC;[110] HPLC with electrochemical detection;[108][111] GC–MS;[74][110] and liquid chromatography coupled to mass spectrometry.[112] Although the determination of psilocin levels in urine can be performed without sample clean-up (i.e., removing potential contaminants that make it difficult to accurately assess concentration), the analysis in plasma or serum requires a preliminary extraction, followed by derivatization of the extracts in the case of GC–MS. A specific immunoassay has also been developed to detect psilocin in whole blood samples.[113] A 2009 publication reported using HPLC to quickly separate forensically important illicit drugs including psilocybin and psilocin, which were identifiable within about half a minute of analysis time.[114] These analytical techniques to determine psilocybin concentrations in body fluids are, however, not routinely available, and not typically used in clinical settings.[24]
History
Early

There is evidence to suggest that psychoactive mushrooms have been used by humans in religious ceremonies for thousands of years. 6,000-year-old pictographs discovered near the Spanish town of Villar del Humo illustrate several mushrooms that have been tentatively identified as Psilocybe hispanica, a hallucinogenic species native to the area.[115]
Archaeological artifacts from Mexico, as well as the so-called Mayan "mushroom stones" of Guatemala have also been interpreted by some scholars as evidence for ritual and ceremonial usage of psychoactive mushrooms in the Mayan and Aztec cultures of Mesoamerica.[73]: 11 In Nahuatl, the language of the Aztecs, the mushrooms were called teonanácatl, or "God's flesh". Following the arrival of Spanish explorers to the New World in the sixteenth century, chroniclers reported the use of mushrooms by the natives for ceremonial and religious purposes. According to the Dominican friar Diego Durán in The History of the Indies of New Spain (published c. 1581), mushrooms were eaten in festivities conducted on the occasion of the accession to the throne of Aztec emperor Moctezuma II in 1502. The Franciscan friar Bernardino de Sahagún wrote of witnessing mushroom usage in his Florentine Codex (published 1545–1590),[116]: 164 and described how some merchants would celebrate upon returning from a successful business trip by consuming mushrooms to evoke revelatory visions.[117] After the defeat of the Aztecs, the Spanish forbade traditional religious practices and rituals that they considered "pagan idolatry", including ceremonial mushroom use. For the next four centuries, the Indians of Mesoamerica hid their use of entheogens from the Spanish authorities.[116]: 165
Although dozens of species of psychedelic mushrooms are found in Europe, there is little documented usage of these species in Old World history besides the use of Amanita muscaria among Siberian peoples.[118][119] The few existing historical accounts about psilocybin mushrooms typically lack sufficient information to allow species identification, and usually refer to the nature of their effects. For example, Flemish botanist Carolus Clusius (1526–1609) described the bolond gomba (crazy mushroom), used in rural Hungary to prepare love potions. English botanist John Parkinson included details about a "foolish mushroom" in his 1640 herbal Theatricum Botanicum.[120]: 10–12 The first reliably documented report of intoxication with Psilocybe semilanceata—Europe's most common and widespread psychedelic mushroom—involved a British family in 1799, who prepared a meal with mushrooms they had picked in London's Green Park.[120]: 16
Modern
American banker and amateur ethnomycologist R. Gordon Wasson and his wife Valentina P. Wasson, a physician, studied the ritual use of psychoactive mushrooms by the native population in the Mazatec village Huautla de Jiménez, Mexico. In 1957, Wasson described the psychedelic visions that he experienced during these rituals in "Seeking the Magic Mushroom", an article published in the popular American weekly Life magazine.[121] Later the same year they were accompanied on a follow-up expedition by French mycologist Roger Heim, who identified several of the mushrooms as Psilocybe species.[122] Heim cultivated the mushrooms in France, and sent samples for analysis to Albert Hofmann, a chemist employed by the Swiss multinational pharmaceutical company Sandoz (now Novartis). Hofmann, who had in 1938 created LSD, led a research group that isolated and identified the psychoactive compounds from Psilocybe mexicana.[123][124] Hofmann was aided in the discovery process by his willingness to ingest mushroom extracts to help verify the presence of the active compounds.[117] He and his colleagues later synthesized a number of compounds chemically related to the naturally occurring psilocybin, to see how structural changes would affect psychoactivity. The new molecules differed from psilocybin in the position of the phosphoryl or hydroxyl group at the top of the indole ring, and in the numbers of methyl groups (CH3) and other additional carbon chains.[25]: 237

Two diethyl analogs (containing two ethyl groups in place of the two methyl groups) of psilocybin and psilocin were synthesized by Hofmann: 4-phosphoryloxy-N,N-diethyltryptamine, called CEY-19, and 4-hydroxy-N,N-diethyltryptamine, called CZ-74. Because their physiological effects last only about three and a half hours (about half as long as psilocybin), they proved more manageable in European clinics using "psycholytic therapy"—a form of psychotherapy involving the controlled use of psychedelic drugs.[25]: 237 Sandoz marketed and sold pure psilocybin under the name Indocybin to physicians and clinicians worldwide.[116]: 166 There were no reports of serious complications when psilocybin was used in this way.[15]
In the early 1960s, Harvard University became a testing ground for psilocybin, through the efforts of Timothy Leary and his associates Ralph Metzner and Richard Alpert (who later changed his name to Ram Dass). Leary obtained synthesized psilocybin from Hofmann through Sandoz pharmaceutical. Some studies, such as the Concord Prison Experiment, suggested promising results using psilocybin in clinical psychiatry.[9][125] According to a 2008 review of safety guidelines in human hallucinogenic research, however, Leary and Alpert's well-publicized termination from Harvard and later advocacy of hallucinogen use "further undermined an objective scientific approach to studying these compounds".[126] In response to concerns about the increase in unauthorized use of psychedelic drugs by the general public, psilocybin and other hallucinogenic drugs suffered negative press and faced increasingly restrictive laws. In the United States, laws were passed in 1966 that prohibited the production, trade, or ingestion of hallucinogenic drugs; Sandoz stopped producing LSD and psilocybin the same year.[77] Further backlash against LSD usage swept psilocybin along with it into the Schedule I category of illicit drugs in 1970. Subsequent restrictions on the use of these drugs in human research made funding for such projects difficult to obtain, and scientists who worked with psychedelic drugs faced being "professionally marginalized".[127]

Despite the legal restrictions on psilocybin use, the 1970s witnessed the emergence of psilocybin as the "entheogen of choice".[128]: 276 This was due in large part to a wide dissemination of information on the topic, which included works such as those by author Carlos Castaneda, and several books that taught the technique of growing psilocybin mushrooms. One of the most popular of this latter group was published in 1976 under the pseudonyms O.T. Oss and O.N. Oeric by Jeremy Bigwood, Dennis J. McKenna, K. Harrison McKenna, and Terence McKenna, entitled Psilocybin: Magic Mushroom Grower's Guide. Over 100,000 copies were sold by 1981.[129] As ethnobiologist Jonathan Ott explains, "These authors adapted San Antonio's technique (for producing edible mushrooms by casing mycelial cultures on a rye grain substrate; San Antonio 1971) to the production of Psilocybe [Stropharia] cubensis. The new technique involved the use of ordinary kitchen implements, and for the first time the layperson was able to produce a potent entheogen in his own home, without access to sophisticated technology, equipment or chemical supplies."[128]: 290 San Antonio's technique describes a method to grow the common edible mushroom Agaricus bisporus[130]
Because of a lack of clarity about laws about psilocybin mushrooms, retailers in the late 1990s and early 2000s commercialized and marketed them in smartshops in the Netherlands and the UK, and online. Several websites[lower-alpha 4] emerged that have contributed to the accessibility of information on description, use, effects and exchange of experiences among users. Since 2001, six EU countries have tightened their legislation on psilocybin mushrooms in response to concerns about their prevalence and increasing usage.[43] In the 1990s, hallucinogens and their effects on human consciousness were again the subject of scientific study, particularly in Europe. Advances in neuropharmacology and neuropsychology, and the availability of brain imaging techniques have provided impetus for using drugs like psilocybin to probe the "neural underpinnings of psychotic symptom formation including ego disorders and hallucinations".[8] Recent studies in the United States have attracted attention from the popular press and thrust psilocybin back into the limelight.[131][132]
Society and culture
Legal status
In the United States, psilocybin (and psilocin) were first subjected to federal regulation by the Drug Abuse Control Amendments of 1965, a product of a bill sponsored by Senator Thomas J. Dodd. The law—passed in July 1965 and effected on February 1, 1966—was an amendment to the federal Food, Drug and Cosmetic Act and was intended to regulate the unlicensed "possession, manufacture, or sale of depressant, stimulant and hallucinogenic drugs".[133]: 25 The statutes themselves, however, did not list the "hallucinogenic drugs" that were being regulated.[133]: 25 Instead, the term "hallucinogenic drugs" was meant to refer to those substances believed to have a "hallucinogenic effect on the central nervous system".[133]: 25
Despite the seemingly strict provisions of the law, many people were exempt from prosecution. The statutes "permit[ted] … people to possess such drugs so long as they were for the personal use of the possessor, [for] a member of his household, or for administration to an animal".[133]: 25 The federal law that specifically banned psilocybin and psilocin was enacted on October 24, 1968. The substances were said to have "a high potential for abuse", "no currently accepted medical use," and "a lack of accepted safety".[133]: 26 On October 27, 1970, both psilocybin and psilocin became classified as Schedule I drugs and were simultaneously labeled "hallucinogens" under a section of the Comprehensive Drug Abuse Prevention and Control Act known as the Controlled Substances Act.[134] Schedule I drugs are illicit drugs that are claimed to have no known therapeutic benefit. Johns Hopkins researchers suggest that if psilocybin clears the current phase III clinical trials, it should be re-categorized to a schedule IV drug such as prescription sleep aids, but with tighter control.[135][136]
The United Nations Convention on Psychotropic Substances (adopted in 1971) requires its members to prohibit psilocybin, and parties to the treaty are required to restrict use of the drug to medical and scientific research under strictly controlled conditions. However, the mushrooms containing the drug were not specifically included in the convention, due largely to pressure from the Mexican government.[137]
Most national drug laws have been amended to reflect the terms of the convention; examples include the UK Misuse of Drugs Act 1971, the US Psychotropic Substances Act of 1978,[134] Australia Poisons Standard (October 2015),[138] the Canadian Controlled Drugs and Substances Act of 1996,[7]: 178–9 and the Japanese Narcotics and Psychotropics Control Law of 2002.[7]: 167–86 The possession and use of psilocybin is prohibited under almost all circumstances, and often carries severe legal penalties.[137]
Possession and use of psilocybin mushrooms, including the bluing species of Psilocybe, is therefore prohibited by extension. However, in many national, state, and provincial drug laws, there has been a great deal of ambiguity about the legal status of psilocybin mushrooms, as well as a strong element of selective enforcement in some places.[139][133]: 25–48 Most US state courts have considered the mushroom a 'container' of the illicit drugs, and therefore illegal. A loophole further complicates the legal situation—the spores of psilocybin mushrooms do not contain the drugs, and are legal to possess in many areas. Jurisdictions that have specifically enacted or amended laws to criminalize the possession of psilocybin mushroom spores include Germany (since 1998),[7] and California, Georgia, and Idaho in the United States. As a consequence, there is an active underground economy involved in the sale of spores and cultivation materials, and an internet-based social network to support the illicit activity.[116]: 177–178
Despite being illegal in many typically Western countries, such as the UK, Australia and some US states, less conservative governments opt to nurture the legal use of psilocybin and other psychedelic drugs. In Amsterdam, Netherlands, authorities provide education and promotion on the safe use of psychedelic drugs, such as psilocybin, in an aim to reduce public harm.[140] Similarly, religious groups like America's Uniao do Vegetal, UDV,[141] use psychedelics in traditional ceremonies, fundamentally contributing to the way people in those communities interact with each other peacefully.[142] Current jurisdiction surrounding psilocybin in Australia fails to address proper grounds for negative impacts,[143] as it has not been found to impact mental health[144] amongst other material constructions of health. Psilocybin have been found for some individuals to have bare minimal harmful effects and have benefited some individuals that suffer from depression. However, because they are illegal even though they offer alternatives to several under treated psychological conditions it is very difficult to research.[136] The criminalisation of possession or use of psilocybin in countries like Australia, US, UK and Japan, causes separation between civilians in a greater sense – those who abide by the law and those who do not and should therefore be incarcerated. Legalisation of psilocybin may help embrace some of the positive impacts of taking the substance, such as “ego dissolution”[141] and may reduce forms of cultural discrimination against traditional owners.[145]
Usage
A 2009 survey found that the number of first-time psilocybin mushroom users in the United States was roughly equivalent to the number of first-time users of cannabis.[137] In Europe, the lifetime use of psychedelic mushroom usage among young adults (15–34 years) range from 0.3% to 14.1%.[146]
In modern Mexico, traditional ceremonial use survives among several indigenous groups, including the Nahuas, the Matlatzinca, the Totonacs, the Mazatecs, Mixes, Zapotecs, and the Chatino. Although hallucinogenic Psilocybe species are abundant in low-lying areas of Mexico, most ceremonial use takes places in mountainous areas of elevations greater than 1,500 meters (4,900 ft). Guzmán suggests this is a vestige of Spanish colonial influence from several hundred years earlier, when mushroom use was persecuted by the Catholic Church.[147]
Natural occurrence
| Species | % psilocybin |
|---|---|
| P. azurescens | 1.78 |
| P. serbica | 1.34 |
| P. semilanceata | 0.98 |
| P. baeocystis | 0.85 |
| P. cyanescens | 0.85 |
| P. tampanensis | 0.68 |
| P. cubensis | 0.63 |
| P. weilii | 0.61 |
| P. hoogshagenii | 0.60 |
| P. stuntzii | 0.36 |
| P. cyanofibrillosa | 0.21 |
| P. liniformans | 0.16 |
Psilocybin is present in varying concentrations in over 200 species of Basidiomycota mushrooms. In a 2000 review on the worldwide distribution of hallucinogenic mushrooms, Gastón Guzmán and colleagues considered these to be distributed amongst the following genera: Psilocybe (116 species), Gymnopilus (14), Panaeolus (13), Copelandia (12), Hypholoma (6), Pluteus (6), Inocybe (6), Conocybe (4), Panaeolina (4), Gerronema (2), and Galerina (1 species).[148] Guzmán increased his estimate of the number of psilocybin-containing Psilocybe to 144 species in a 2005 review. The majority of these are found in Mexico (53 species), with the remainder distributed in the United States and Canada (22), Europe (16), Asia (15), Africa (4), and Australia and associated islands (19).[149] The diversity of psilocybin mushrooms is reported to have been increased by horizontal transfer of the psilocybin gene cluster between unrelated mushroom species.[150][95] In general, psilocybin-containing species are dark-spored, gilled mushrooms that grow in meadows and woods of the subtropics and tropics, usually in soils rich in humus and plant debris.[83]: 5 Psilocybin mushrooms occur on all continents, but the majority of species are found in subtropical humid forests.[148] Psilocybe species commonly found in the tropics include P. cubensis and P. subcubensis. P. semilanceata—considered by Guzmán to be the world's most widely distributed psilocybin mushroom[151]—is found in Europe, North America, Asia, South America, Australia and New Zealand, but is entirely absent from Mexico.[149] Although the presence or absence of psilocybin is not of much use as a chemotaxonomical marker at the familial level or higher, it is used to classify taxa of lower taxonomic groups.[152]


Both the caps and the stems contain the psychoactive compounds, although the caps consistently contain more. The spores of these mushrooms do not contain psilocybin or psilocin.[103][154][155] The total potency varies greatly between species and even between specimens of a species collected or grown from the same strain.[156] Because most psilocybin biosynthesis occurs early in the formation of fruit bodies or sclerotia, younger, smaller mushrooms tend to have a higher concentration of the drug than larger, mature mushrooms.[157] In general, the psilocybin content of mushrooms is quite variable (ranging from almost nothing to 2.5% of the dry weight)[158][25]: 248 and depends on species, strain, growth and drying conditions, and mushroom size.[73]: 36–41, 52 Cultivated mushrooms have less variability in psilocybin content than wild mushrooms.[139] The drug is more stable in dried than fresh mushrooms; dried mushrooms retain their potency for months or even years,[73]: 51–5 while mushrooms stored fresh for four weeks contain only traces of the original psilocybin.[3]
The psilocybin contents of dried herbarium specimens of Psilocybe semilanceata in one study were shown to decrease with the increasing age of the sample: collections dated 11, 33, or 118 years old contained 0.84%, 0.67%, and 0.014% (all dry weight), respectively.[159] Mature mycelia contain some psilocybin, while young mycelia (recently germinated from spores) lack appreciable amounts.[160] Many species of mushrooms containing psilocybin also contain lesser amounts of the analog compounds baeocystin and norbaeocystin,[73]: 38 chemicals thought to be biogenic precursors.[7]: 170 Although most species of psilocybin-containing mushrooms bruise blue when handled or damaged due to the oxidization of phenolic compounds, this reaction is not a definitive method of identification or determining a mushroom's potency.[156][73]: 56–58
Research

Psilocybin has been a subject of preliminary research since the early 1960s, when the Harvard Psilocybin Project evaluated the potential therapeutic value of psilocybin for personality disorders.[161] Beginning in the 2000s decade, research on anxiety disorders, major depression, and various addictions was conducted.[162][116]: 179–81 Psilocybin has been tested for its potential for developing prescription drugs to treat drug dependence, anxiety, or mood disorders.[163][164] Psilocybin and LSD are under preliminary research as possible psychoplastogens.[165][166][167]
In 2018–2019, the United States Food and Drug Administration (FDA) granted Breakthrough Therapy Designation for psilocybin-assisted therapy for treatment-resistant depression[168] and major depressive disorder, a review process enabling the FDA to implement an expedited review if clinical research results for psilocybin use in treating depression are compelling.[169] A 2021 review found use of psilocybin was associated with reduced intensity of depression symptoms.[170]
See also
- List of entheogens
- O-Acetylpsilocin
- Psychoactive drug
- Psychedelic experience
- Psychedelic microdosing
- Psychedelic plants
- Soma (drink)
- Psychoplastogen
Notes
- ↑ Percentages are derived from a non-blind clinical study of 30 individuals who were given a dosage of 8–12 milligrams of psilocybin; from Passie (2002),[15] citing Quentin (1960).[16]
- ↑ One of the reported fatalities, that of a 22-year-old French man who died in 1993,[51] was later challenged in the literature by Jochen Gartz and colleagues, who concluded "the few reported data concerning the victim are insufficient to exclude other possible causes of the fatality".[52]
- ↑ Subjective effects are "feelings, perceptions, and moods personally experienced by an individual"; they are often assessed using methods of self-report, including questionnaires. Behavioral effects, in contrast, can be observed directly.[69]
- ↑ The EMCDDA lists the general-purpose websites Erowid, Lycaeum, Mycotopia, The Shroomery, MushroomJohn and The Entheogen Review. Regional sites focusing on hallucinogenic mushrooms listed were Copenhagen Mushroom Link (Denmark), Champis (France), Daath (Hungary), Delysid (Spain), Enteogeneos (Portugal), Kouzelné houbičky (Czech Republic), Norshroom (Norway), Planetahongo (Spain), Svampinfo (Sweden), and Taikasieniforum (Finland). It also listed Magic-Mushrooms.net. The report detailed several additional sites selling spore prints in 2006, but noted that many of these had ceased operation.
References
- ↑ "Psilocybine – Compound Summary". PubChem. National Library of Medicine. Archived from the original on September 25, 2012. Retrieved December 4, 2011.
- ↑ "Psilocybine". PubChem, US National Library of Medicine. 27 August 2022. Archived from the original on 9 October 2022. Retrieved 29 August 2022.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 van Amsterdam J, Opperhuizen A, van den Brink W (2011). "Harm potential of magic mushroom use: a review" (PDF). Regulatory Toxicology and Pharmacology. 59 (3): 423–429. doi:10.1016/j.yrtph.2011.01.006. PMID 21256914. Archived from the original (PDF) on January 9, 2015.
- ↑ "Hallucinogenic Drug Psilocybin Eases Existential Anxiety in People With Life-Threatening Cancer". Johns Hopkins. December 1, 2016. Archived from the original on April 7, 2021. Retrieved April 9, 2019.
- ↑ Griffiths RR, Johnson MW, Richards WA, Richards BD, McCann U, Jesse R (June 15, 2011). "Psilocybin occasioned mystical-type experiences: Immediate and persisting dose-related effects". Johns Hopkins. 218 (4): 649–665. doi:10.1007/s00213-011-2358-5. PMC 3308357. PMID 21674151.
- 1 2 3 Hasler F, Grimberg U, Benz MA, Huber T, Vollenweider FX (2004). "Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study" (PDF). Psychopharmacology. 172 (2): 145–156. doi:10.1007/s00213-003-1640-6. PMID 14615876. S2CID 15263504. Archived (PDF) from the original on April 18, 2020. Retrieved April 10, 2019.
- 1 2 3 4 5 6 Ballesteros S, Ramón MF, Iturralde MJ, Martínez-Arrieta R (2006). "Natural sources of drugs of abuse: magic mushrooms". In Cole SM (ed.). New Research on Street Drugs. New York, New York: Nova Science Publishers. pp. 167–186. ISBN 978-1-59454-961-8. Archived from the original on April 4, 2017. Retrieved February 27, 2016.
- 1 2 Studerus E, Kometer M, Hasler F, Vollenweider FX (2011). "Acute, subacute and long-term subjective effects of psilocybin in healthy humans: a pooled analysis of experimental studies". Journal of Psychopharmacology. 25 (11): 1434–1452. doi:10.1177/0269881110382466. PMID 20855349. S2CID 22923427.
- 1 2 Leary T, Litwin GH, Metzner R (1963). "Reactions to psilocybin administered in a supportive environment". Journal of Nervous and Mental Disease. 137 (6): 561–573. doi:10.1097/00005053-196312000-00007. PMID 14087676. S2CID 39777572.
- ↑ Berge JT (1999). "Breakdown or breakthrough? A history of European research into drugs and creativity". Journal of Creative Behavior. 33 (4): 257–276. doi:10.1002/j.2162-6057.1999.tb01406.x. ISSN 0022-0175.
- 1 2 MacLean KA, Johnson MW, Griffiths RR (2011). "Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness". Journal of Psychopharmacology. 25 (11): 1453–1461. doi:10.1177/0269881111420188. PMC 3537171. PMID 21956378.
- ↑ Griffiths RR, Johnson MW, Richards WA, Richards BD, Jesse R, MacLean KA, et al. (January 2018). "Psilocybin-occasioned mystical-type experience in combination with meditation and other spiritual practices produces enduring positive changes in psychological functioning and in trait measures of prosocial attitudes and behaviors". Journal of Psychopharmacology. 32 (1): 49–69. doi:10.1177/0269881117731279. PMC 5772431. PMID 29020861.
- ↑ "Psilocybin (from magic mushrooms) plus meditation and spiritual training leads to lasting changes in positive traits". January 19, 2018. Archived from the original on February 21, 2019. Retrieved February 21, 2019.
- ↑ Frecska E, Luna LE (2006). "The adverse effects of hallucinogens from intramural perspective" (PDF). Neuropsychopharmacologia Hungarica. 8 (4): 189–200. PMID 17211054. Archived (PDF) from the original on September 16, 2018. Retrieved December 27, 2011.
- 1 2 3 4 5 6 7 8 9 Passie T, Seifert J, Schneider U, Emrich HM (2002). "The pharmacology of psilocybin". Addiction Biology. 7 (4): 357–364. doi:10.1080/1355621021000005937. PMID 14578010. S2CID 12656091.
- ↑ Quentin AM (1960). La Psilocybine en Psychiatrie Clinique et Experimentale [Psilocybin in Clinical and Experimental Psychiatry] (PhD thesis) (in français). Paris, France: Paris University Medical Dissertation.
- ↑ See for example:
- Isbell H (1959). "Comparison of the reactions induced by psilocybin and LSD-25 in man". Psychopharmacologia. 1 (1): 29–38. doi:10.1007/BF00408109. PMID 14405870. S2CID 19508675.
- Hollister LE, Prusmack JJ, Paulsen A, Rosenquist N (1960). "Comparison of three psychotropic drugs (psilocybin, JB-329, and IT-290) in volunteer subjects". Journal of Nervous and Mental Disease. 131 (5): 428–434. doi:10.1097/00005053-196011000-00007. PMID 13715375. S2CID 8255131.
- Malitz S, Esecover H, Wilkens B, Hoch PH (1960). "Some observations on psilocybin, a new hallucinogen, in volunteer subjects". Comprehensive Psychiatry. 1: 8–17. doi:10.1016/S0010-440X(60)80045-4. PMID 14420328.
{{cite journal}}: CS1 maint: url-status (link) - Rinkel M, Atwell CR, Dimascio A, Brown J (1960). "Experimental psychiatry. V. Psilocybine, a new psychotogenic drug". New England Journal of Medicine. 262 (6): 295–297. doi:10.1056/NEJM196002112620606. PMID 14437505.
- Parashos AJ (1976). "The psilocybin-induced "state of drunkenness" in normal volunteers and schizophrenics". Behavioral Neuropsychiatry. 8 (1–12): 83–86. PMID 1052267.
- ↑ Heimann H (1994). "Experience of time and space in model psychoses". In Pletscher A, Ladewig D (eds.). 50 Years of LSD. Current Status and Perspectives of Hallucinogens. New York, New York: The Parthenon Publishing Group. pp. 59–66. ISBN 978-1-85070-569-7.
- 1 2 Wittmann M, Carter O, Hasler F, Cahn BR, Grimberg U, Spring P, Hell D, Flohr H, Vollenweider FX (2007). "Effects of psilocybin on time perception and temporal control of behaviour in humans". Journal of Psychopharmacology. 21 (1): 50–64. doi:10.1177/0269881106065859. PMID 16714323. S2CID 3165579.
- ↑ Wackermann J, Wittmann M, Hasler F, Vollenweider FX (2008). "Effects of varied doses of psilocybin on time interval reproduction in human subjects". Neuroscience Letters. 435 (1): 51–55. doi:10.1016/j.neulet.2008.02.006. PMID 18325673. S2CID 22789140.
- ↑ Carter OL, Burr DC, Pettigrew JD, Wallis GM, Hasler F, Vollenweider FX (2005). "Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors" (PDF). Journal of Cognitive Neuroscience. 17 (10): 1497–1508. doi:10.1162/089892905774597191. PMID 16269092. S2CID 9790150. Archived (PDF) from the original on August 16, 2019. Retrieved August 16, 2019.
- ↑ Harrington DL, Haaland KY (1999). "Neural underpinnings of temporal processing: a review of focal lesion, pharmacological, and functional imaging research". Reviews in the Neurosciences. 10 (2): 91–116. doi:10.1515/REVNEURO.1999.10.2.91. PMID 10658954. S2CID 25960626.
- 1 2 Coull JT, Cheng RK, Meck WH (2011). "Neuroanatomical and neurochemical substrates of timing". Neuropsychopharmacology. 36 (1): 3–25. doi:10.1038/npp.2010.113. PMC 3055517. PMID 20668434.
- 1 2 Attema-de Jonge ME, Portier CB, Franssen EJ (2007). "Automutilatie na gebruik van hallucinogene paddenstoelen" [Automutilation after consumption of hallucinogenic mushrooms]. Nederlands Tijdschrift voor Geneeskunde (in Nederlands). 151 (52): 2869–2872. PMID 18257429.
- 1 2 3 4 Stafford PJ (1992). Psychedelics Encyclopedia (3rd ed.). Berkeley, California: Ronin Publishing. ISBN 978-0-914171-51-5.
- ↑ "Drug Addictions, Hallucinogens and Shamanism: the View from Anthropology - Document - Gale Academic OneFile". Archived from the original on August 23, 2021. Retrieved August 23, 2021.
- 1 2 3 4 Batchelder T (2001). "Drug Addictions, Hallucinogens and Shamanism: the View from Anthropology". Drug Addictions, Hallucinogens and Shamanism. Townsend Letter for Doctors and Patients. 217: 74–77. Archived from the original on October 19, 2021. Retrieved August 23, 2021 – via Gale Academic OneFile.
- ↑ Leary T (2007). The psychedelic experience : a manual based on the Tibetan book of the dead. Ralph Metzner, Ram Dass, activeth century Karma-gliṅ-pa. New York: Citadel Press. ISBN 978-0-8065-1652-3. OCLC 318713242. Archived from the original on October 19, 2021. Retrieved August 23, 2021.
- ↑ Batchelder, Tim (1 July 2001). "Drug Addictions, Hallucinogens and Shamanism: the View from Anthropology". Townsend Letter for Doctors and Patients. pp. 74–74. Archived from the original on 8 February 2022. Retrieved 10 November 2022.
- ↑ Hendricks PS (October 2020). "Psilocybin-assisted group therapy: A new hope for demoralization". EClinicalMedicine. 27: 100557. doi:10.1016/j.eclinm.2020.100557. PMC 7549063. PMID 33073220.
- ↑ Winkelman MJ (2007). "Therapeutic bases of psychedelic medicines: psychointegrative effects". In Winkelman MJ, Roberts TB (eds.). Psychedelic Medicine: New Evidence for Hallucinogenic Substances as Treatments. Vol. 1. Westport, Connecticut: Praeger. pp. 1–19. ISBN 978-0-275-99024-4.
- ↑ McKenna T (1993). Food of the Gods: The Search for the Original Tree of Knowledge. A Radical History of Plants, Drugs, and Human Evolution. New York, New York: Bantam Books. ISBN 978-0-553-37130-7.
- ↑ James W (1902). The Varieties of Religious Experience. New York, New York: Simon & Schuster. ISBN 978-0-684-84297-4.
- ↑ Metzner R (1998). "Hallucinogenic drugs and plants in psychotherapy and shamanism" (PDF). Journal of Psychoactive Drugs. 30 (4): 333–341. CiteSeerX 10.1.1.509.4769. doi:10.1080/02791072.1998.10399709. PMID 9924839. Archived (PDF) from the original on September 21, 2017. Retrieved October 26, 2017.
- ↑ Pahnke WN, Richards W (1966). "Implications of LSD and experimental mysticism". Journal of Religion and Health. 5 (3): 175–208. doi:10.1007/BF01532646. PMID 24424798. S2CID 18464414.
- ↑ Pahnke WN (1966). "Drugs and mysticism". International Journal of Parapsychology. 8 (2): 295–315.
- ↑ Griffiths R, Richards W, Johnson M, McCann U, Jesse R (2008). "Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later" (PDF). Journal of Psychopharmacology. 22 (6): 621–632. doi:10.1177/0269881108094300. PMC 3050654. PMID 18593735. Archived from the original (PDF) on July 22, 2008. Retrieved July 3, 2008.
- ↑ Smith H (2000). Cleansing the Doors of Perception: The Religious Significance of Entheogenic Plants and Chemicals. New York, New York: Jeremy P. Tarcher/Putnam. p. 101. ISBN 978-1-58542-034-6.
- 1 2 Doblin R (1991). "Pahnke's "Good Friday Experiment": a long-term follow-up and methodological critique". Journal of Transpersonal Psychology. 23 (1): 1–25.
- ↑ Richards WA (2008). "The phenomenology and potential religious import of states of consciousness facilitated by psilocybin". Archive for the Psychology of Religion. 30 (1): 189–199. doi:10.1163/157361208X317196. S2CID 144969540.
- 1 2 Carhart-Harris RL, Nutt DJ (2010). "User perceptions of the benefits and harms of hallucinogenic drug use: a web-based questionnaire study". Journal of Substance Abuse. 15 (4): 283–300. doi:10.3109/14659890903271624. S2CID 56427651.
- ↑ "Paul McHugh reviews Don Lattin's "The Harvard Psychedelic Club."". commentarymagazine.com. April 1, 2010. Archived from the original on April 10, 2019. Retrieved April 10, 2019.
- 1 2 Hillebrand J, Olszewski D, Sedefov R (2006). Hallucinogenic Mushrooms: An Emerging Trend Case Study (PDF) (Report). Lisbon, Portugal: European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). ISBN 92-9168-249-7. Archived (PDF) from the original on March 29, 2012. Retrieved September 8, 2011.
- ↑ Pagliaro LA, Pagliaro AM (2012). Handbook of Child and Adolescent Drug and Substance Abuse: Pharmacological, Developmental, and Clinical Considerations (2nd ed.). Hoboken, New Jersey: John Wiley & Sons. p. 199. ISBN 978-0-470-63906-1. Archived from the original on April 3, 2017. Retrieved February 27, 2016.
- ↑ Schaefer C (2001). Drugs During Pregnancy and Lactation: Handbook of Prescription Drugs and Comparative Risk Assessment. Amsterdam, The Netherlands: Elsevier. p. 222. ISBN 978-0-444-50763-1. Archived from the original on April 3, 2017. Retrieved February 27, 2016.
- ↑ Gable RS (2006). "Acute toxicity of drugs versus regulatory status". In Fish JM (ed.). Drugs and Society: U.S. Public Policy. Lanham, Maryland: Rowman & Littlefield. pp. 149–162, Table 7.1 "Safety Ratio and Dependence Potential of Psychoactive Drugs". ISBN 978-0-7425-4245-7. Archived from the original on January 7, 2012.
- ↑ Reinert JP, Colunga K, Etuk A, Richardson V, Dunn RL (August 2020). "Management of overdoses of salvia, kratom, and psilocybin mushrooms: a literature review". Expert Rev Clin Pharmacol (Review). 13 (8): 847–856. doi:10.1080/17512433.2020.1794811. PMID 32648791. S2CID 220472473.
- 1 2 O'Neil MJ, Smith A, Heckelman PE, Obenchain JR, Gallipeau JR, D'Arecca MA, eds. (2001). The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (13th ed.). Whitehouse Station, New Jersey: Merck. p. 1419. ISBN 978-0-911910-13-1.
- ↑ Gable RS (2004). "Comparison of acute lethal toxicity of commonly abused psychoactive substances" (PDF). Addiction. 99 (6): 686–696. doi:10.1111/j.1360-0443.2004.00744.x. PMID 15139867. Archived (PDF) from the original on August 10, 2006. Retrieved November 16, 2011.
- ↑ Strassman R, Wojtowicz S, Luna LE, Frecska E (2008). Inner Paths to Outer Space: Journeys to Alien Worlds through Psychedelics and Other Spiritual Technologies. Rochester, Vermont: Park Street Press. p. 147. ISBN 978-1-59477-224-5. Archived from the original on April 4, 2017. Retrieved February 27, 2016.
- ↑ Gérault A, Picart D (1996). "Intoxication mortelle à la suite de la consommation volontaire et en groupe de champignons hallucinogènes" [Fatal poisoning after a group of people voluntarily consumed hallucinogenic mushrooms]. Bulletin de la Société Mycologique de France (in français). 112: 1–14.
- ↑ Gartz J, Samorini G, Festi F (1996). "On the presumed French case of fatality caused by ingestion of Liberty Caps". Eluesis. 6: 40–41. Archived from the original on April 5, 2012.
- ↑ Peden NR, Pringle SD, Crooks J (1982). "The problem of psilocybin mushroom abuse". Human Toxicology. 1 (4): 417–424. doi:10.1177/096032718200100408. PMID 7173927. S2CID 7453965.
- 1 2 Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D (1998). "Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action" (PDF). NeuroReport. 9 (17): 3897–3902. doi:10.1097/00001756-199812010-00024. PMID 9875725. S2CID 37706068. Archived from the original (PDF) on 2019-03-03.
- ↑ Hyde C, Glancy P, Omerod P, Hall D, Taylor GS (1978). "Abuse of indigenous psilocybin mushrooms: a new fashion and some psychiatric complications". British Journal of Psychiatry. 132 (6): 602–604. doi:10.1192/bjp.132.6.602. PMID 566144. S2CID 20020560.
- ↑ Mack RB (1983). "Phenomenally phunny phungi – psilocybin toxicity". New Castle Medical Journal. 44 (10): 639–640. PMID 6580536.
- ↑ Simeon D (2004). "Depersonalisation disorder: a contemporary overview". CNS Drugs. 18 (6): 343–354. doi:10.2165/00023210-200418060-00002. PMID 15089102. S2CID 18506672.
- ↑ Nielen RJ, van der Heijden FM, Tuinier S, Verhoeven WM (January 2004). "Khat and mushrooms associated with psychosis". The World Journal of Biological Psychiatry. 5 (1): 49–53. doi:10.1080/15622970410029908. PMID 15048636. S2CID 44321700.
- ↑ Geyer MA (1998). "Behavioral studies of hallucinogenic drugs in animals: implications for schizophrenia research". Pharmacopsychiatry. 31 (S2): 73–79. doi:10.1055/s-2007-979350. PMID 9754837.
- 1 2 Vollenweider FX, Geyer MA (2001). "A systems model of altered consciousness: integrating natural and drug-induced psychoses". Brain Research Bulletin. 56 (5): 495–507. doi:10.1016/S0361-9230(01)00646-3. PMID 11750795. S2CID 230298.
- ↑ Geyer MA, Vollenweider FX (2008). "Serotonin research: contributions to understanding psychoses". Trends in Pharmacological Sciences. 29 (9): 445–453. doi:10.1016/j.tips.2008.06.006. PMID 19086254.
- ↑ Myers LS, Watkins SS, Carter TJ (1998). "Flashbacks in theory and practice" (PDF). The Heffter Review of Psychedelic Research. 1: 51–57. Archived (PDF) from the original on September 27, 2011. Retrieved August 14, 2011.
- ↑ Nicholas LG, Ogame K (2006). Psilocybin Mushroom Handbook: Easy Indoor and Outdoor Cultivation. Oakland, California: Quick American Archives. p. 164. ISBN 978-0-932551-71-9. Archived from the original on April 4, 2017. Retrieved February 27, 2016.
- ↑ Passie T, Halpern JH, Stichtenoth, Emrich HM, Hintzen A (2008). "The pharmacology of lysergic acid diethyamide: a review". CNS Neuroscience & Therapeutics. 14 (4): 295–314. doi:10.1111/j.1755-5949.2008.00059.x. PMC 6494066. PMID 19040555.
- 1 2 Halberstadt AL, Geyer MA (2011). "Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens". Neuropharmacology. 61 (3): 364–381. doi:10.1016/j.neuropharm.2011.01.017. PMC 3110631. PMID 21256140.
- ↑ Chen CY, Storr CL, Anthony JC (2008). "Early-onset drug use and risk for drug dependence problems". Addictive Behaviors. 34 (3): 319–322. doi:10.1016/j.addbeh.2008.10.021. PMC 2677076. PMID 19022584.
- ↑ van Amsterdam J, Opperhuizen A, Koeter M, van den Brink W (2010). "Ranking the harm of alcohol, tobacco and illicit drugs for the individual and the population". European Addiction Research. 16 (4): 202–207. doi:10.1159/000317249. PMID 20606445. S2CID 207669364.
- ↑ Nutt DJ, King LA, Phillips LD (2010). "Drug harms in the UK: a multicriteria decision analysis". Lancet. 376 (9752): 1558–1565. CiteSeerX 10.1.1.690.1283. doi:10.1016/S0140-6736(10)61462-6. PMID 21036393. S2CID 5667719.
- ↑ Karch SB (2007). Pharmacokinetics and Pharmacodynamics of Abused Drugs. Boca Raton, Florida: CRC Press. p. 148. ISBN 978-1-4200-5458-3. Archived from the original on April 4, 2017. Retrieved February 27, 2016.
- ↑ "PDSP Ki Database". PDSP. Archived from the original on May 9, 2021. Retrieved 20 January 2020.
- ↑ Adams Jr JD (2009). "Chemical interactions with pyramidal neurons in layer 5 of the cerebral cortex: control of pain and anxiety". Current Medicinal Chemistry. 16 (27): 3476–3279. doi:10.2174/092986709789057626. PMID 19799545.
- ↑ Mason NL, Kuypers KP, Müller F, Reckweg J, Tse DH, Toennes SW, et al. (November 2020). "Me, myself, bye: regional alterations in glutamate and the experience of ego dissolution with psilocybin". Neuropsychopharmacology. 45 (12): 2003–2011. doi:10.1038/s41386-020-0718-8. PMC 7547711. PMID 32446245.
- 1 2 3 4 5 6 7 Stamets P (1996). Psilocybin Mushrooms of the World: An Identification Guide. Berkeley, California: Ten Speed Press. ISBN 978-0-89815-839-7.
- 1 2 Grieshaber AF, Moore KA, Levine B (2001). "The detection of psilocin in human urine". Journal of Forensic Sciences. 46 (3): 627–630. doi:10.1520/JFS15014J. PMID 11373000.
- ↑ Hasler F, Bourquin D, Brenneisen R, Vollenweider FX (2002). "Renal excretion profiles of psilocin following oral administration of psilocybin: a controlled study in man". Journal of Pharmaceutical and Biomedical Analysis. 30 (2): 331–339. doi:10.1016/S0731-7085(02)00278-9. PMID 12191719.
- ↑ Meyer MR, Maurer HH (2011). "Absorption, distribution, metabolism and excretion pharmacogenomics of drugs of abuse". Pharmacogenomics. 12 (2): 215–233. doi:10.2217/pgs.10.171. PMID 21332315.
- 1 2 Matsushima Y, Eguchi F, Kikukawa T, Matsuda T (2009). "Historical overview of psychoactive mushrooms". Inflammation and Regeneration. 29 (1): 47–58. doi:10.2492/inflammregen.29.47.
- ↑ Baselt RC (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, California: Biomedical Publications. pp. 1346–1348. ISBN 978-0-9626523-7-0.
- ↑ Beck O, Helander A, Karlson-Stiber C, Stephansson N (1998). "Presence of phenylethylamine in hallucinogenic Psilocybe mushroom: possible role in adverse reactions". Journal of Analytical Toxicology. 22 (1): 45–49. doi:10.1093/jat/22.1.45. PMID 9491968.
- ↑ van Amsterdam J, Talhout R, Vleeming W, Opperhuizen A (2006). "Contribution of monoamine oxidase (MAO) inhibition to tobacco and alcohol addiction". Life Sciences. 79 (21): 1969–1973. doi:10.1016/j.lfs.2006.06.010. PMID 16884739.
- ↑ Gilbert J, Şenyuva H (2009). Bioactive Compounds in Foods. John Wiley & Sons. p. 120. ISBN 978-1-4443-0229-5. Archived from the original on April 4, 2017. Retrieved February 27, 2016.
- ↑ Azmitia EC (2010). "Evolution of serotonin: sunlight to suicide". In Müller CP, Jacobs BL (eds.). Handbook of the Behavioral Neurobiology of Serotonin. London, UK: Academic Press. p. 7. ISBN 978-0-12-374634-4. Archived from the original on April 4, 2017. Retrieved February 27, 2016.
- 1 2 3 4 Wurst M, Kysilka R, Flieger M (2002). "Psychoactive tryptamines from Basidiomycetes". Folia Microbiologica. 47 (1): 3–27. doi:10.1007/BF02818560. PMID 11980266. S2CID 31056807.
- 1 2 "Psilocybine". Hazardous Substances Data Bank. U.S. National Library of Medicine. Archived from the original on August 13, 2018. Retrieved November 21, 2011.
- 1 2 Anastos N, Barnett NW, Pfeffer FM (2006). "Investigation into the temporal stability of aqueous standard solutions of psilocin and psilocybin using high performance liquid chromatography". Science & Justice. 46 (2): 91–96. doi:10.1016/S1355-0306(06)71579-9. PMID 17002211.
- 1 2 Shirota O, Hakamata W, Goda Y (2003). "Concise large-scale synthesis of psilocin and psilocybin, principal hallucinogenic constituents of "magic mushroom"". Journal of Natural Products. 66 (6): 885–887. CiteSeerX 10.1.1.925.6269. doi:10.1021/np030059u. PMID 12828485.
- ↑ Troxler F, Seeman F, Hofmann A (1959). "Abwandlungsprodukte von Psilocybin und Psilocin. 2. Mitteilung über synthetische Indolverbindungen" [Modified products of psilocybin and psilocin. 2. Report on synthetic indole compounds]. Helvetica Chimica Acta (in Deutsch). 42 (6): 2073–2103. doi:10.1002/hlca.19590420638.
- ↑ Hofmann A, Frey A, Ott H, Petrzilka T, Troxler F (1958). "Konstitutionsaufklärung und Synthese von Psilocybin" [The composition and synthesis of psilocybin]. Cellular and Molecular Life Sciences (in Deutsch). 14 (11): 397–399. doi:10.1007/BF02160424. PMID 13609599. S2CID 33692940.
- ↑ Nichols DE, Frescas S (1999). "Improvements to the synthesis of psilocybin and a facile method for preparing the o-acetyl prodrug of psilocin". Synthesis. 1999 (6): 935–938. CiteSeerX 10.1.1.690.8071. doi:10.1055/s-1999-3490.
- ↑ Sherwood, A. M.; Kargbo, R. B.; Kaylo, K. W.; Cozzi, N. V.; Meisenheimer, P.; Kaduk, J. A. (2022-01-01). "Psilocybin: crystal structure solutions enable phase analysis of prior art and recently patented examples". Acta Crystallographica Section C: Structural Chemistry. 78 (1): 36–55. doi:10.1107/S2053229621013164. ISSN 2053-2296. PMC 8725723. PMID 34982048. Archived from the original on 2022-03-16. Retrieved 2022-11-03.
- ↑ Kargbo, Robert B.; Sherwood, Alexander; Walker, Andrew; Cozzi, Nicholas V.; Dagger, Raymond E.; Sable, Jessica; O’Hern, Kelsey; Kaylo, Kristi; Patterson, Tura; Tarpley, Gary; Meisenheimer, Poncho (2020-07-14). "Direct Phosphorylation of Psilocin Enables Optimized cGMP Kilogram-Scale Manufacture of Psilocybin". ACS Omega. 5 (27): 16959–16966. doi:10.1021/acsomega.0c02387. PMC 7364850. PMID 32685866.
- ↑ Agurell S, Nilsson JL (1968). "Biosynthesis of psilocybin. Part II. Incorporation of labelled tryptamine derivatives". Acta Chemica Scandinavica. 22 (4): 1210–1218. doi:10.3891/acta.chem.scand.22-1210. PMID 5750023.
- ↑ Chilton WS, Bigwood J, Jensen RE (1979). "Psilocin, bufotenine and serotonin: historical and biosynthetic observations". Journal of Psychedelic Drugs. 11 (1–2): 61–69. doi:10.1080/02791072.1979.10472093. PMID 392119.
- 1 2 3 Fricke J, Blei F, Hoffmeister D (September 2017). "Enzymatic synthesis of psilocybin". Angewandte Chemie. 56 (40): 12352–12355. doi:10.1002/anie.201705489. PMID 28763571.
- 1 2 Reynolds HT, Vijayakumar V, Gluck-Thaler E, Korotkin HB, Matheny PB, Slot JC (April 2018). "Horizontal gene cluster transfer increased hallucinogenic mushroom diversity". Evolution Letters. 2 (2): 88–101. doi:10.1002/evl3.42. PMC 6121855. PMID 30283667.
- ↑ Satyanarayana M (7 October 2019). "Modified E. coli pump out psilocybin". Chemical & Engineering News. 97 (39): 11. doi:10.1021/cen-09739-scicon9. S2CID 208747979. Archived from the original on December 3, 2019. Retrieved December 3, 2019.
- ↑ Technical University of Denmark (2020-04-16). "Psychedelic compound from magic mushrooms produced in yeast". phys.org. Archived from the original on April 30, 2020. Retrieved 2020-05-02.
- ↑ Milne N (2021-01-15). "Magic Yeasts and How to Make Them Produce Psilocybin". MIND Foundation. Archived from the original on January 15, 2021. Retrieved 2021-01-17.
- ↑ Jenkins AJ (2003). "Hallucinogens". In Levine B (ed.). Principles of Forensic Toxicology (2nd ed.). Washington, D.C.: American Association for Clinical Chemistry Press. p. 281. ISBN 978-1-890883-87-4. Archived from the original on April 3, 2017. Retrieved February 27, 2016.
- ↑ Cole MD (2003). The Analysis of Controlled Substances. New York, Chichester: John Wiley and Sons. pp. 132–133. ISBN 978-0-471-49252-8.
- ↑ Bresinsky A, Besl H (1989). A Colour Atlas of Poisonous Fungi: A Handbook for Pharmacists, Doctors, and Biologists. London, UK: Manson Publishing. p. 113. ISBN 978-0-7234-1576-3. Archived from the original on April 4, 2017. Retrieved February 27, 2016.
- ↑ Kamata T, Katagi M, Tsuchihashi H (2010). "Metabolism and toxicological analyses of hallucinogenic tryptamine analogues being abused in Japan". Forensic Toxicology. 28 (1): 1–8. doi:10.1007/s11419-009-0087-9. S2CID 45118221.
- 1 2 Keller T, Schneider A, Regenscheit P, Dirnhofer R, Rücker T, Jaspers J, Kisser W (1999). "Analysis of psilocybin and psilocin in Psilocybe subcubensis Guzmán by ion mobility spectrometry and gas chromatography-mass spectrometry". Forensic Science International. 99 (2): 93–105. doi:10.1016/S0379-0738(98)00168-6. PMID 10077856.
- ↑ Pedersen-Bjergaard S, Sannes E, Rasmussen K, Tonneson F (1997). "Determination of psilocybin in Psilocybe semilanceata by capillary zone electrophoresis". Journal of Chromatography. 694 (2): 375–381. doi:10.1016/S0378-4347(97)00127-8. PMID 9252052.
- ↑ Lee RE (1985). "A technique for the rapid isolation and identification of psilocin from psilocin/psilocybin-containing mushrooms". Journal of Forensic Sciences. 30 (3): 931–941. doi:10.1520/JFS11028J. PMID 4040953.
- ↑ Wurst M, Kysilka R, Koza T (1992). "Analysis and isolation of indole alkaloids of fungi by high-performance liquid chromatography". Journal of Chromatography. 593 (1–2): 201–208. doi:10.1016/0021-9673(92)80287-5.
- ↑ Saito K, Toyo'oka T, Fukushima T, Kato M, Shirota O, Goda Y (2004). "Determination of psilocin in magic mushrooms and rat plasma by liquid chromatography with fluorimetry and electrospray ionization mass spectrometry". Analytica Chimica Acta. 527 (2): 149–156. doi:10.1016/j.aca.2004.08.071.
- 1 2 Lindenblatt H, Kramer E, Holzmann-Erens P, Gouzoulis-Mayfrank E, Kovar K (1998). "Quantitation of psilocin in human plasma by high performance liquid chromatography and electrochemical detection: comparison of liquid-liquid extraction with automated on-line solid-phase extraction". Journal of Chromatography. 709 (2): 255–263. doi:10.1016/S0378-4347(98)00067-X. PMID 9657222.
- ↑ Rodriguez-Cruz SE (2005). "Analysis and characterization of psilocybin and psilocin using liquid chromatography-electrospray ionization mass spectrometry (LC-ESI-MS) with collision-induced-dissociation (CID) and source-induced dissociation (SID)". Microgram Journal. 3 (3–4): 175–82. Archived from the original on April 29, 2011.
- 1 2 Sticht G, Käferstein H (2000). "Detection of psilocin in body fluids". Forensic Science International. 113 (1): 403–407. doi:10.1016/S0379-0738(00)00213-9. PMID 10978655.
- ↑ Kysilka R (1990). "Determination of psilocin in rat urine by high-performance liquid chromatography with electrochemical detection". Journal of Chromatography. 534: 287–290. doi:10.1016/S0378-4347(00)82176-3. PMID 2094720.
- ↑ Kamata T, Nishikawa M, Katagi M, Tsuchihashi H (2003). "Optimized glucuronide hydrolysis for the detection of psilocin in human urine samples". Journal of Chromatography B. 792 (2): 421–427. doi:10.1016/j.jchromb.2003.08.030. PMID 14581081.
- ↑ Albers C, Köhler H, Lehr M, Brinkmann B, Beike J (2004). "Development of a psilocin immunoassay for serum and blood samples". International Journal of Legal Medicine. 118 (6): 326–331. doi:10.1007/s00414-004-0469-9. PMID 15526212. S2CID 11249439.
- ↑ Lurie I, Li L (2009). "Use of high-temperature liquid chromatography with sub-2 µm particle C18 columns for the analysis of seized drugs". Journal of Liquid Chromatography & Related Technologies. 32 (17–20): 2615–2626. doi:10.1080/10826070903245516. S2CID 96753191. Archived from the original on May 3, 2021. Retrieved August 24, 2020.
- ↑ Akers BP, Ruiz JF, Piper A, Ruck CA (2011). "A prehistoric mural in Spain depicting neurotropic Psilocybe mushrooms?". Economic Botany. 65 (2): 121–128. doi:10.1007/s12231-011-9152-5. S2CID 3955222.
- 1 2 3 4 5 Marley G (2010). "Psilocybin: gateway to the soul or just a good high?". Chanterelle Dreams, Amanita Nightmares: The Love, Lore, and Mystique of Mushrooms. White River Junction, Vermont: Chelsea Green Publishing. pp. 163–184. ISBN 978-1-60358-214-8.
- 1 2 Hofmann A (1980). "The Mexican relatives of LSD". LSD: My Problem Child. New York, New York: McGraw-Hill. pp. 49–71. ISBN 978-0-07-029325-0.
- ↑ Nyberg H (1992). "Religious use of hallucinogenic fungi: A comparison between Siberian and Mesoamerican Cultures". Karstenia. 32 (2): 71–80. doi:10.29203/ka.1992.294.
- ↑ Wasson RG (1968). Soma: Divine Mushroom of Immortality. Harcourt Brace Jovanovick. p. 161. ISBN 978-0-88316-517-1.
- 1 2 Gartz J (1997). Magic Mushrooms Around the World. Los Angeles, California: LIS Publications. ISBN 978-0-9653399-0-2.
- ↑ Wasson RG (May 13, 1957). "Seeking the magic mushroom". Life. pp. 101–120. ISSN 0024-3019. Archived from the original on April 3, 2017. Retrieved February 27, 2016.
- ↑ Heim R (1957). "Notes préliminaires sur les agarics hallucinogènes du Mexique" [Preliminary notes on the hallucination-producing agarics of Mexico]. Revue de Mycologie (in français). 22 (1): 58–79.
- ↑ Hofmann A, Heim R, Brack A, Kobel H (1958). "Psilocybin, ein psychotroper Wirkstoff aus dem mexikanischen Rauschpilz Psilocybe mexicana Heim" [Psilocybin, a psychotropic drug from the Mexican magic mushroom Psilocybe mexicana Heim]. Experientia (in Deutsch). 14 (3): 107–109. doi:10.1007/BF02159243. PMID 13537892. S2CID 42898430.
- ↑ Hofmann A, Heim R, Brack A, Kobel H, Frey A, Ott H, Petrzilka T, Troxler F (1959). "Psilocybin und Psilocin, zwei psychotrope Wirkstoffe aus mexikanischen Rauschpilzen" [Psilocybin and psilocin, two psychotropic substances in Mexican magic mushrooms]. Helvetica Chimica Acta (in Deutsch). 42 (5): 1557–1572. doi:10.1002/hlca.19590420518.
- ↑ Leary T, Metzner R, Presnell M, Weil G, Schwitzgebel R, Kinne S (1965). "A new behavior change program using psilocybin". Psychotherapy: Theory, Research & Practice. 2 (2): 61–72. doi:10.1037/h0088612.
- ↑ Johnson MW, Richards WA, Griffiths RR (2008). "Human hallucinogen research: guidelines for safety" (PDF). Journal of Psychopharmacology. 22 (6): 603–620. doi:10.1177/0269881108093587. PMC 3056407. PMID 18593734. Archived from the original (PDF) on November 20, 2017. Retrieved November 20, 2017.
- ↑ Griffiths RR, Grob CS (2010). "Hallucinogens as medicine" (PDF). Scientific American. 303 (6): 77–79. Bibcode:2010SciAm.303f..76G. doi:10.1038/scientificamerican1210-76. Archived from the original (PDF) on October 3, 2011. Retrieved July 25, 2011.
- 1 2 Ott J (1993). Pharmacotheon: Entheogenic Drugs, their Plant Sources and History. Kennewick, Washington: Natural Products Company. ISBN 978-0-9614234-3-8.
- ↑ Oeric OT, Os ON (1991). Psilocybin: Magic Mushroom Grower's Guide (2nd ed.). San Francisco, California: Quick American Archives. ISBN 978-0-932551-06-1.
- ↑ San Antonio JP (1971). "A laboratory method to obtain fruit from cased grain spawn of the cultivated mushroom, Agaricus bisporus". Mycologia. 63 (1): 16–21. doi:10.2307/3757680. JSTOR 3757680. PMID 5102274. Archived from the original on September 23, 2015. Retrieved September 7, 2011.
- ↑ Keim B (July 1, 2008). "Psilocybin study hints at rebirth of hallucinogen research". Wired.com. Archived from the original on August 3, 2011. Retrieved August 8, 2011.
- ↑ Miller G (July 1, 2008). "A very memorable trip". sciencemag.org. Archived from the original on August 13, 2018. Retrieved August 8, 2011.
- 1 2 3 4 5 6 Boire RG (2002). Sacred Mushrooms and the Law. Berkeley, California: Ronin Publishing. ISBN 978-1-57951-061-9.
- 1 2 "List of psychotropic substances under international control" (PDF) (23rd ed.). Vienna, Austria: International Narcotics Control Board. August 2003. Archived from the original (PDF) on December 5, 2005.
- ↑ "Hopkins researchers recommend reclassifying psilocybin, the drug in 'magic' mushrooms, from schedule I to schedule IV". Johns Hopkins. September 26, 2018. Archived from the original on April 25, 2019. Retrieved April 25, 2019.
- 1 2 Johnson MW, Griffiths RR, Hendricks PS, Henningfield JE (November 2018). "The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act". Neuropharmacology. 142: 143–166. doi:10.1016/j.neuropharm.2018.05.012. PMC 6791528. PMID 29753748.
- 1 2 3 Bone E (2011). Mycophilia: Revelations from the Weird World of Mushrooms. New York, New York: Rodale. pp. 257–258. ISBN 978-1-60529-407-0. Archived from the original on April 4, 2017. Retrieved February 27, 2016.
- ↑ "Misuse of Drugs Act 1981" (PDF). 2015-11-18. Archived from the original (PDF) on December 22, 2015 – via www.slp.wa.gov.au.
- 1 2 "Drug profiles: Hallucinogenic mushrooms". European Monitoring Centre for Drugs and Drug Addiction. September 19, 2011. Archived from the original on November 27, 2011. Retrieved December 4, 2011.
- ↑ Hardon A, van Schipstal I, Berning M, Mishra S, Murray H, Mandler T, Kamps D, Hymans TD (December 2020). "Caring for "Hassle‐Free Highs" in Amsterdam". Anthropology and Humanism. 45 (2): 212–22. doi:10.1111/anhu.12298. S2CID 228997721.
- 1 2 Pollan M (2018). Como mudar sua mente [How to kill your mind] (in Portuguese). Editora Intrinseca. ISBN 9788551004173.
{{cite book}}: CS1 maint: unrecognized language (link) - ↑ Shipley M (November 2015). Psychedelic mysticism: Transforming consciousness, religious experiences, and voluntary peasants in postwar America. Lexington Books. ISBN 978-1-4985-0910-7.
- ↑ Submission to the Western Australian Inquiry Into Alternative Approaches to Reducing Illicit Drug Use and Its Effects on the Community (PDF) (Report). Australian Psychedelic Society Inc. 30 January 2019. Archived (PDF) from the original on August 23, 2021. Retrieved August 23, 2021.
- ↑ Johansen PØ, Krebs TS (March 2015). "Psychedelics not linked to mental health problems or suicidal behavior: a population study". Journal of Psychopharmacology. 29 (3): 270–9. doi:10.1177/0269881114568039. PMID 25744618. S2CID 2025731.
- ↑ Rodd R (September 2018). "It's all you! Australian ayahuasca drinking, spiritual development, and immunitary individualism". Critique of Anthropology. 38 (3): 325–45. doi:10.1177/0308275X18775818. S2CID 149858755.
- ↑ European Monitoring Centre for Drugs and Drug Addiction (November 2011). Annual report 2011: the state of the drugs problem in Europe (PDF) (Report). Luxembourg: Publications Office of the European Union. doi:10.2810/44330. ISBN 978-92-9168-470-0. Archived (PDF) from the original on December 3, 2011. Retrieved December 4, 2011.
- ↑ Guzmán G (2008). "Hallucinogenic mushrooms in Mexico: an overview". Economic Botany. 62 (3): 404–412. doi:10.1007/s12231-008-9033-8. S2CID 22085876.
- 1 2 Guzmán G, Allen JW, Gartz J (2000). "A worldwide geographical distribution of the neurotropic fungi, an analysis and discussion" (PDF). Annali del Museo Civico di Rovereto: Sezione Archeologia, Storia, Scienze Naturali. 14: 189–280. Archived (PDF) from the original on February 5, 2018. Retrieved October 19, 2021.
- 1 2 Guzmán G (2005). "Species diversity of the genus Psilocybe (Basidiomycotina, Agaricales, Strophariaceae) in the world mycobiota, with special attention to hallucinogenic properties". International Journal of Medicinal Mushrooms. 7 (1–2): 305–331. doi:10.1615/intjmedmushr.v7.i12.280.
- ↑ Yong E (24 August 2017). "How Mushrooms Became Magic". The Atlantic. Archived from the original on March 16, 2018. Retrieved March 15, 2018.
- ↑ Guzmán G (1983). The Genus Psilocybe: A Systematic Revision of the Known Species Including the History, Distribution, and Chemistry of the Hallucinogenic Species. Beihefte Zur Nova Hedwigia. Heft 74. Vaduz, Liechtenstein: J. Cramer. pp. 361–362. ISBN 978-3-7682-5474-8.
- ↑ Saupe SG (1981). "Occurrence of psilocybin/psilocin in Pluteus salicinus (Plutaceae)". Mycologia. 73 (4): 871–874. doi:10.2307/3759505. JSTOR 3759505. Archived from the original on March 10, 2017. Retrieved August 29, 2011.
- ↑ Guzmán G, Allen JW, Gartz J (1998). "A worldwide geographical distribution of the neurotropic fungi, an analysis and discussion" (PDF). Annali del Museo Civico di Rovereto. 14: 207. Archived from the original (PDF) on June 26, 2010. Retrieved September 17, 2017.
- ↑ Wurst M, Semerdžieva M, Vokoun J (1984). "Analysis of psychotropic compounds in fungi of the genus Psilocybe by reversed-phase high performance liquid chromatography". Journal of Chromatography A. 286: 229–235. doi:10.1016/S0021-9673(01)99190-3.
- ↑ Kysilka R, Wurst M (1989). "High-performance liquid chromatographic determination of some psychotropic indole derivatives". Journal of Chromatography. 464 (2): 434–437. doi:10.1016/s0021-9673(00)94264-x. PMID 2722990.
- 1 2 Bigwood J, Beug MW (1982). "Variation of psilocybin and psilocin levels with repeated flushes (harvests) of mature sporocarps of Psilocybe cubensis (Earle) Singer". Journal of Ethnopharmacology. 5 (3): 287–291. doi:10.1016/0378-8741(82)90014-9. PMID 7201054.
- ↑ Gartz J (1992). "New aspects of the occurrence, chemistry and cultivation of European hallucinogenic mushrooms". Supplemento Agli Annali dei Musei Civici di Rovereto Sezione Archeologica, Storia e Scienze Naturali. 8: 107–124.
- ↑ Laussmann, Tim; Meier-Giebing, Sigrid (2010-02-25). "Forensic analysis of hallucinogenic mushrooms and khat (Catha edulis Forsk) using cation-exchange liquid chromatography". Forensic Science International. 195 (1–3): 160–164. doi:10.1016/j.forsciint.2009.12.013. ISSN 1872-6283. PMID 20047807. Archived from the original on 2022-06-12. Retrieved 2022-11-03.
- ↑ Ohenoja E, Jokiranta J, Mäkinen T, Kaikkonen A, Airaksinen MM (1987). "The occurrence of psilocybin and psilocin in Finnish fungi". Journal of Natural Products. 50 (4): 741–744. doi:10.1021/np50052a030. PMID 3430170.
- ↑ Gross ST (2000). "Detecting psychoactive drugs in the developmental stages of mushrooms" (PDF). Journal of Forensic Sciences. 45 (3): 527–537. doi:10.1520/JFS14725J. PMID 10855955. S2CID 38006957. Archived from the original (PDF) on 2020-11-16.
- ↑ Wark C, Galliher JF (2009). "Timothy Leary, Richard Alpert (Ram Dass) and the changing definition of psilocybin". The International Journal on Drug Policy. 21 (3): 234–239. doi:10.1016/j.drugpo.2009.08.004. PMID 19744846.
- ↑ Brown D (July 11, 2006). "Drug's mystical properties confirmed". Washington Post. Archived from the original on May 4, 2011. Retrieved September 12, 2011.
- ↑ Dos Santos RG, Osório FL, Crippa JA, Riba J, Zuardi AW, Hallak JE (June 2016). "Antidepressive, anxiolytic, and antiaddictive effects of ayahuasca, psilocybin and lysergic acid diethylamide (LSD): a systematic review of clinical trials published in the last 25 years". Therapeutic Advances in Psychopharmacology. 6 (3): 193–213. doi:10.1177/2045125316638008. PMC 4910400. PMID 27354908.
- ↑ Ross S, Bossis A, Guss J, Agin-Liebes G, Malone T, Cohen B, et al. (December 2016). "Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial". Journal of Psychopharmacology. 30 (12): 1165–1180. doi:10.1177/0269881116675512. PMC 5367551. PMID 27909164.
- ↑ Olson, David E (2018-09-19). "Psychoplastogens: A Promising Class of Plasticity-Promoting Neurotherapeutics". Journal of Experimental Neuroscience. 12: 1179069518800508. doi:10.1177/1179069518800508. ISSN 1179-0695. PMC 6149016. PMID 30262987.
- ↑ Vargas, Maxemiliano V.; Meyer, Retsina; Avanes, Arabo A.; Rus, Mark; Olson, David E. (2021). "Psychedelics and Other Psychoplastogens for Treating Mental Illness". Frontiers in Psychiatry. 12: 727117. doi:10.3389/fpsyt.2021.727117. ISSN 1664-0640. PMC 8520991. PMID 34671279.
- ↑ de Vos, Cato M. H.; Mason, Natasha L.; Kuypers, Kim P. C. (2021). "Psychedelics and Neuroplasticity: A Systematic Review Unraveling the Biological Underpinnings of Psychedelics". Frontiers in Psychiatry. 12: 1575. doi:10.3389/fpsyt.2021.724606. ISSN 1664-0640. PMC 8461007. PMID 34566723.
- ↑ "COMPASS Pathways Receives FDA Breakthrough Therapy Designation for Psilocybin Therapy for Treatment-resistant Depression". Compass Pathways. Archived from the original on December 4, 2018. Retrieved 2018-12-03.
- ↑ Richard Staines (2 December 2019). "FDA tags psilocybin drug as clinical depression Breakthrough Therapy". Pharmaphorum. Archived from the original on September 7, 2021. Retrieved 7 September 2021.
- ↑ Więckiewicz G, Stokłosa I, Piegza M, Gorczyca P, Pudlo R (August 2021). "Lysergic Acid Diethylamide, Psilocybin and Dimethyltryptamine in Depression Treatment: A Systematic Review". Pharmaceuticals. 14 (8): 793. doi:10.3390/ph14080793. PMC 8399008. PMID 34451890.
- Griffiths RR, Richards WA, McCann U, Jesse R (2006). "Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance" (PDF). Psychopharmacology. 187 (3): 268–283. doi:10.1007/s00213-006-0457-5. PMID 16826400. S2CID 7845214. Archived from the original (PDF) on November 9, 2011.
- "Press release: Griffiths psilocybin". Johns Hopkins Medicine. July 11, 2006. Archived from the original on July 16, 2011. Retrieved July 12, 2006.
External links
Psilocybin Investigator`s Brochure Archived 2021-08-13 at the Wayback Machine Usona Institute, 2021
| Identifiers: |
|---|