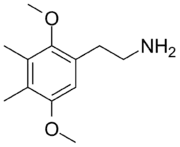
2C-G
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-(2,5-Dimethoxy-3,4-dimethylphenyl)ethan-1-amine | |||
| Identifiers | |||
CAS Number |
|||
3D model (JSmol) |
|||
| ChEMBL | |||
| ChemSpider | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula |
C12H19NO2 | ||
| Molar mass | 209.289 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
2C-G is a psychedelic phenethylamine of the 2C family. First synthesized by Alexander Shulgin,[1] it is sometimes used as an entheogen. It has structural and pharmacodynamic properties similar to 2C-D and Ganesha. Like many of the phenethylamines in PiHKAL, 2C-G and its homologs have only been taken by Shulgin and a small test group, making it difficult to ensure completeness when describing effects.
Chemistry
2C-G is 3,4-dimethyl-2,5-dimethoxyphenethylamine, with the formula C
12H
19NO
2.
Dosage and effects
In Shulgin's book PiHKAL, the dosage range is listed as 20 to 35 mg.[1] Effects are similar to the related Ganesha, and are extremely long lasting; the duration is 18–30 hours. Visual effects are muted or absent, and it is described in PiHKAL as an "insight-enhancer".[1] Unlike other members of the 2C family, 2C-G is nearly as potent as its amphetamine cousin.
Homologs
Several homologs of 2C-G were also synthesized by Shulgin. These include 2C-G-3, 2C-G-5, and 2C-G-N. Some, such as 2C-G-1, 2C-G-2, 2C-G-4, and 2C-G-6 are possible to synthesize in principle but impossible or extraordinarily difficult to do so in practice.
| 2C-G-1 | CAS: not available The synthesis of this compound has not been reported |
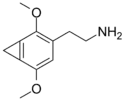 2C-G-1 |
| 2C-G-2 | CAS: not available The synthesis of this compound has not been reported |
 2C-G-2 |
| 2C-G-3 | CAS: 207740-19-0 Dosage: 16–25 mg |
 2C-G-3 |
| 2C-G-4 | CAS: 952006-59-6 Synthesized but not tested. |
 2C-G-4 |
| 2C-G-5 | CAS: 207740-20-3 Dosage: 10–16 mg |
 2C-G-5 |
| 2C-G-6 | CAS: not available The synthesis of this compound has not been reported |
 2C-G-6 |
| 2C-G-N | CAS: 207740-21-4 Dosage: 20–40 mg |
Legal status
Canada
As of October 31, 2016; 2C-G is a controlled substance (Schedule III) in Canada.[2]
United Kingdom
2C-G and all other compounds featuring in PiHKAL are Class A drugs in the United Kingdom.
United States
2C-G and all of its homologs are unscheduled in the United States, but possession and sales of 2C-G and homologs might be prosecuted under the Federal Analog Act because of their close structural similarities to 2C-B, which is a Schedule I controlled substance.
See also
References
External links
- , , , PiHKAL entries on the homologues
- 2C-G Entry in PiHKAL • info
- 2C-G-3 Entry in PiHKAL • info
- 2C-G-4 Entry in PiHKAL • info
- 2C-G-5 Entry in PiHKAL • info
- 2C-G-N Entry in PiHKAL • info


