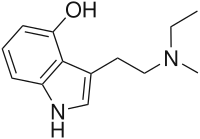
4-HO-MET
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3-{2-[Ethyl(methyl)amino]ethyl}-1H-indol-4-ol | |
| Other names
4-hydroxy-N-methyl-N-ethyltryptamine; metocin; 3-{2-[ethyl(methyl)amino]ethyl}-1H-indol-4-ol | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C13H18N2O |
| Molar mass | 218.300 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
4-HO-MET (4-hydroxy-N-methyl-N-ethyltryptamine, metocin, or methylcybin), is a lesser-known psychedelic drug. It is a structural− and functional analog of psilocin as well as the 4-hydroxyl analog of methylethyltryptamine (MET). 4-HO-MET was first synthesized by Alexander Shulgin. In his book TiHKAL (Tryptamines I Have Known and Loved), the dosage is listed as 10-20 mg.[1] 4-HO-MET produces psilocin-like distortion of color, sound, and form. Very little data exists about the pharmacological properties, metabolism, and toxicity of 4-HO-MET. There have been no reports of deaths from 4-HO-MET, even though people have reported taking doses up to 150 mg,[2] more than an order of magnitude above the effective dose.[3]
Effects
Users report similar effects to psilocin, including mydriasis, closed and open eye visuals, euphoria, time dilation and general change in thought processes. These effects occur in a wavelike pattern such as that of psilocybin with near-normal perception and high effect varying rapidly. The effects last for about 4–6 hours.[4][5]
Pharmacology
Pharmacokinetics
| Binding Sites | Binding Affinity Ki (μM)[6] |
|---|---|
| 5-HT1A | 0.228 |
| 5-HT2A | 0.057 |
| 5-HT2C | 0.141 |
| D1 | >25 |
| D2 | 4 |
| D3 | 6.7 |
| α1A | 9.7 |
| α2A | 2.4 |
| TAAR1 | 3.1 |
| H1 | 0.82 |
| SERT | 0.2 |
| DAT | >26 |
| NET | 13 |
Drug prohibition laws
Sweden
Sveriges riksdag added 4-HO-MET to schedule I ("substances, plant materials and fungi which normally do not have medical use") as narcotics in Sweden as of May 1, 2012, published by Medical Products Agency in their regulation LVFS 2012:6 listed as 4-HO-MET 3-[2-[etyl(metyl)amino]etyl]-1H-indol-4-ol.[7]
United Kingdom
4-HO-MET is a class A drug in the UK, as a result of the tryptamine catch-all clause.
United States
4-HO-MET is not scheduled at the federal level in the United States, but it is possible that it could be considered an analogue of psilocin, in which case purchase, sale, or possession could be prosecuted under the Federal Analogue Act.
See also
References
- ↑ Shulgin, Alexander; Shulgin, Ann (September 1997). TiHKAL: The Continuation. Berkeley, California: Transform Press. ISBN 0-9630096-9-9. OCLC 38503252.
- ↑ "Erowid Experience Vaults / Cosmic Mind Orgy". Erowid. 2008-07-22. Retrieved 2014-02-25.
- ↑ "Search Results : Erowid Experience Vaults". www.erowid.org. Retrieved 6 April 2018.
- ↑ "4-HO-MET Effects". Erowid. Retrieved 2014-02-25.
- ↑ "Heaven and Hell—A Phenomenological Study of Recreational Use of 4-HO-MET in Sweden. Kjellgren A. & Soussan C., 2011" (PDF). J Psychoactive Drugs. Retrieved 2019-08-16.
- ↑ Rickli A.; Moning O.D.; Hoener M.C.; Liechti M.E. (2016). "Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens" (PDF). European Neuropsychopharmacology. 26 (8): 1327–1337. doi:10.1016/j.euroneuro.2016.05.001. PMID 27216487. S2CID 6685927.
- ↑ "Föreskrifter om ändring i Läkemedelsverkets föreskrifter (LVFS 2011:10) om förteckningar över narkotika;" (PDF) (in Swedish). Elanders Sverige AB. 2012-04-20. Archived from the original (PDF) on 2013-09-28. Retrieved 2014-02-25.