FGIN-143
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
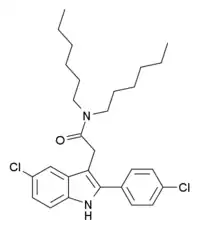
| Formula | C28H36Cl2N2O |
| Molar mass | 487.51 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
FGIN-1-43 is an anxiolytic drug which acts as a selective agonist at the peripheral benzodiazepine receptor, also known as the mitochondrial 18 kDa translocator protein or TSPO. It is thought to produce anxiolytic effects by stimulating steroidogenesis of neuroactive steroids such as allopregnanolone, and is several times more potent than the related drug FGIN-127.[1][2][3][4]
References
- ↑ Romeo E, Auta J, Kozikowski AP, Ma D, Papadopoulos V, Puia G, et al. (September 1992). "2-Aryl-3-indoleacetamides (FGIN-1): a new class of potent and specific ligands for the mitochondrial DBI receptor (MDR)". The Journal of Pharmacology and Experimental Therapeutics. 262 (3): 971–8. PMID 1326631.
- ↑ Kozikowski AP, Ma D, Brewer J, Sun S, Costa E, Romeo E, Guidotti A (October 1993). "Chemistry, binding affinities, and behavioral properties of a new class of "antineophobic" mitochondrial DBI receptor complex (mDRC) ligands". Journal of Medicinal Chemistry. 36 (20): 2908–20. doi:10.1021/jm00072a010. PMID 8411007.
- ↑ Costa E, Auta J, Guidotti A, Korneyev A, Romeo E (June 1994). "The pharmacology of neurosteroidogenesis". The Journal of Steroid Biochemistry and Molecular Biology. 49 (4–6): 385–9. doi:10.1016/0960-0760(94)90284-4. PMID 8043504.
- ↑ Guillon J, Boulouard M, Lelong V, Dallemagne P, Rault S, Jarry C (November 2001). "Synthesis and preliminary behavioural evaluation in mice of new 3-aryl-3-pyrrol-1-ylpropanamides, analogues of FGIN-1-27 and FGIN-1-43". The Journal of Pharmacy and Pharmacology. 53 (11): 1561–8. doi:10.1211/0022357011777945. PMID 11732760.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.