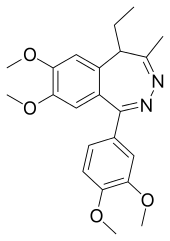
Tofisopam
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 6-(3,4-Dimethoxyphenyl)-2-ethyl-9,10-dimethoxy-3-methyl-4,5-diazabicyclo[5.4.0]undeca-3,5,7,9,11-pentaene |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 3 hours[1][2] |
| Excretion | Renal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.040.823 |
| Chemical and physical data | |
| Formula | C22H26N2O4 |
| Molar mass | 382.5 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Tofisopam[3] (Emandaxin, Grandaxin, Sériel) is an anxiolytic that is marketed in several European countries.[4] Chemically, it is a 2,3-benzodiazepine. Unlike other anxiolytic benzodiazepines (which are generally 1,4- or 1,5-substituted) however, tofisopam does not have anticonvulsant, sedative,[5] skeletal muscle relaxant, motor skill-impairing or amnestic[6] properties. While it may not be an anticonvulsant in and of itself, it has been shown to enhance the anticonvulsant action of classical 1,4-benzodiazepines (such as diazepam) and muscimol, but not sodium valproate, carbamazepine, phenobarbital, or phenytoin.[7][8] Tofisopam is indicated for the treatment of anxiety and alcohol withdrawal, and is prescribed in a dosage of 50–300 mg per day divided into three doses. Peak plasma levels are attained two hours after an oral dose. Tofisopam is not reported as causing dependence to the same extent as other benzodiazepines, but is still recommended to be prescribed for a maximum of 12 weeks.
Tofisopam is not approved for sale in the United States or Canada. However, Vela Pharmaceuticals of New Jersey is developing the D-enantiomer (dextofisopam) as a treatment for irritable bowel syndrome,[9] with moderate efficacy demonstrated in clinical trials so far.[10]
Tofisopam is also claimed to be a PDE10A inhibitor, which may provide an alternative mechanism of action for its various therapeutic effects, and this action has been proposed to make tofisopam potentially useful as a treatment for schizophrenia.[11]
Tofisopam has been shown to act as an inhibitor of the liver enzyme CYP3A4,[12][13] and some researches suspect that this could cause dangerous drug interactions with other medications metabolised by this enzyme,[14][15] although the clinical significance of these findings remains unclear.
References
- ↑ Klebovich I, Abermann M (March 1993). "[Pharmacokinetics and metabolism of tofizopam (Grandaxin)]". Acta Pharm Hung (in Hungarian). 63 (2): 83–90. PMID 8100113.
- ↑ Rundfeldt C, Socała K, Wlaź P (November 2010). "The atypical anxiolytic drug, tofisopam, selectively blocks phosphodiesterase isoenzymes and is active in the mouse model of negative symptoms of psychosis". J Neural Transm (Vienna). 117 (11): 1319–25. doi:10.1007/s00702-010-0507-3. PMC 2993883. PMID 20967473.
- ↑ DE Patent 2122070
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. p. 1041. ISBN 978-3-88763-075-1.
- ↑ Bond A, Lader M (1982). "A comparison of the psychotropic profiles of tofisopam and diazepam". European Journal of Clinical Pharmacology. 22 (2): 137–42. doi:10.1007/BF00542458. PMID 6124424. S2CID 30776062.
- ↑ Seppälä T, Palva E, Mattila MJ, Korttila K, Shrotriya RC (1980). "Tofisopam, a novel 3,4-benzodiazepine: multiple-dose effects on psychomotor skills and memory. Comparison with diazepam and interactions with ethanol". Psychopharmacology. 69 (2): 209–18. doi:10.1007/BF00427652. PMID 6109345. S2CID 24063885.
- ↑ Saano V (1986). "Tofizopam selectively increases the action of anticonvulsants". Medical Biology. 64 (4): 201–6. PMID 3023768.
- ↑ Petócz L (March 1993). "[Pharmacologic effects of tofizopam (Grandaxin)]". Acta Pharmaceutica Hungarica. 63 (2): 79–82. PMID 8100112.
- ↑ Vela Pharmaceuticals (2005). "Vela Announces Positive Phase 2 Results for Dextofisopam in Treating Irritable Bowel Syndrome - IBS: Results Show Effects of Dextofisopam Both in Women and in Men". VelaPharm - News. Archived from the original on May 2, 2005. Retrieved 21 February 2006.
- ↑ Leventer SM, Raudibaugh K, Frissora CL, Kassem N, Keogh JC, Phillips J, Mangel AW (January 2008). "Clinical trial: dextofisopam in the treatment of patients with diarrhoea-predominant or alternating irritable bowel syndrome". Alimentary Pharmacology & Therapeutics. 27 (2): 197–206. doi:10.1111/j.1365-2036.2007.03566.x. PMID 17973974. S2CID 8557111.
- ↑ Nielsen EB, Kehler J, Nielsen J, Brøsen P. Use of Tofisopam as a PDE10A inhibitor. WIPO Patent WO/2007/082546
- ↑ "Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers". FDA. 26 May 2021.
- ↑ Tóth M, Bajnógel J, Egyed A, Drabant S, Tömlo J, Klebovich I (2005). "[Effect of tofisopam on CYP3A4 enzyme activity on human recombinant 3A4 supersome]". Acta Pharmaceutica Hungarica. 75 (4): 195–8. PMID 16711396.
- ↑ Drabant S, Tóth M, Bereczki A, Bajnógel J, Tömlö J, Klebovich I (July 2006). "Effect of tofisopam on the single-oral-dose pharmacokinetics and pharmacodynamics of the cyp3a4 probe drug alprazolam". European Journal of Clinical Pharmacology. 62 (7): 587–8. doi:10.1007/s00228-006-0160-9. PMID 16791582. S2CID 32545296.
- ↑ Tóth M, Drabant S, Varga B, Végso G, Cseh A, Szentpéteri I, Klebovich I (January 2008). "Tofisopam inhibits the pharmacokinetics of CYP3A4 substrate midazolam". European Journal of Clinical Pharmacology. 64 (1): 93–4. doi:10.1007/s00228-007-0397-y. PMID 17989974. S2CID 35022772.