Alprazolam triazolobenzophenone
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
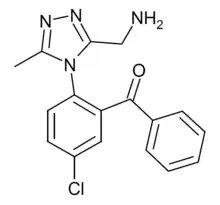
| Formula | C17H15ClN4O |
| Molar mass | 326.8 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Alprazolam triazolobenzophenone is a chemical compound which can be both a synthetic precursor and a prodrug for the benzodiazepine derivative alprazolam. At neutral pH it readily cyclizes to alprazolam, while in acidic conditions alprazolam undergoes a ring-opening reaction back to the triazolobenzophenone. A series of related acyl derivatives was researched in the 1980s as injectable water-soluble prodrugs of alprazolam,[1] but were never developed for medical use. Subsequently, this compound has been detected as a designer drug, first being identified from a seizure in Spain in March 2014.[2]
See also
References
- ↑ Cho MJ, Sethy VH, Haynes LC (August 1986). "Sequentially labile water-soluble prodrugs of alprazolam". Journal of Medicinal Chemistry. 29 (8): 1346–50. doi:10.1021/jm00158a004. PMID 3016261.
- ↑ "Novel Benzodiazepines. A review of the evidence of use and harms of Novel Benzodiazepines" (PDF). Advisory Council on the Misuse of Drugs. April 2020.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.