Flunitrazolam
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
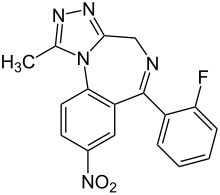
| Formula | C17H12FN5O2 |
| Molar mass | 337.307 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Flunitrazolam (FNTZ, Flunazolam) is a triazolobenzodiazepine (TBZD), which are benzodiazepine (BZD) derivatives, that has been sold online as a designer drug, and is a potent hypnotic and sedative drug similar to related compounds such as flunitrazepam, clonazolam and flubromazolam. It was first definitively identified and reported to the EMCDDA Early Warning System, by an analytical laboratory in Germany in October 2016,[1] and had not been described in the scientific or patent literature before this.[2] It is the triazole analogue of Flunitrazepam (Rohypnol). The addition of the triazole ring to the scaffold increases potency significantly, this is evident as flunitrazolam is reported anecdotally to be active in the microgram level.[3][4]
See also
References
- ↑ "Europol 2016 Annual Report on the implementation of Council Decision 2005/387/JHA" (PDF).
- ↑ Cornett EM, Novitch MB, Brunk AJ, Davidson KS, Menard BL, Urman RD, Kaye AD (June 2018). "New benzodiazepines for sedation". Best Practice & Research. Clinical Anaesthesiology. 32 (2): 149–164. doi:10.1016/j.bpa.2018.06.007. PMID 30322456.
- ↑ Ameline A, Richeval C, Gaulier JM, Raul JS, Kintz P (July 2018). "Characterization of Flunitrazolam, a New Designer Benzodiazepine, in Oral Fluid After a Controlled Single Administration". Journal of Analytical Toxicology. 42 (6): e58–e60. doi:10.1093/jat/bky012. PMID 29462316.
- ↑ Ameline A, Richeval C, Gaulier JM, Raul JS, Kintz P (February 2019). "Detection of the designer benzodiazepine flunitrazolam in urine and preliminary data on its metabolism". Drug Testing and Analysis. 11 (2): 223–229. doi:10.1002/dta.2480. PMID 30109775.