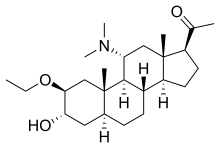
Minaxolone
 | |
| Clinical data | |
|---|---|
| Other names | 11α-(Dimethylamino)-2β-ethoxy-3α-hydroxy-5α-pregnan-20-one |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C25H43NO3 |
| Molar mass | 405.623 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Minaxolone (CCI-12923) is a neuroactive steroid which was developed as a general anesthetic but was withdrawn before registration due to toxicity seen with long-term administration in rats, and hence was never marketed.[1][2][3] It is a positive allosteric modulator of the GABAA receptor,[4] as well as, less potently, a positive allosteric modulator of the glycine receptor.[4][5]
Chemistry
See also
References
- ↑ C.R. Ganellin; David J. Triggle (21 November 1996). Dictionary of Pharmacological Agents. CRC Press. pp. 1358–. ISBN 978-0-412-46630-4.
- ↑ J. G. Bovill; Michael B. Howie (1999). Clinical Pharmacology for Anaesthetists. W.B. Saunders. ISBN 978-0-7020-2167-1.
- ↑ Hugh C. Hemmings; Philip M. Hopkins (2006). Foundations of Anesthesia: Basic Sciences for Clinical Practice. Elsevier Health Sciences. pp. 305–. ISBN 978-0-323-03707-5.
- 1 2 Giovanni Biggio; Robert H. Purdy (2001). Neurosteroids and Brain Function. Academic Press. pp. 196–. ISBN 978-0-12-366846-2.
- ↑ Weir CJ, Ling AT, Belelli D, Wildsmith JA, Peters JA, Lambert JJ (May 2004). "The interaction of anaesthetic steroids with recombinant glycine and GABAA receptors". Br J Anaesth. 92 (5): 704–11. CiteSeerX 10.1.1.519.3591. doi:10.1093/bja/aeh125. PMID 15033889.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.