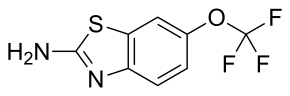
Riluzole
 | |
 | |
| Names | |
|---|---|
| Trade names | Rilutek, Tiglutik, Exservan, others |
IUPAC name
| |
| Clinical data | |
| Main uses | Amyotrophic lateral sclerosis[1] |
| Side effects | Weakness, liver problems[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a696013 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 60±18%[3] |
| Protein binding | 97%[3] |
| Metabolism | Liver (CYP1A2)[3] |
| Elimination half-life | 9–15 hours[3] |
| Excretion | Urine (90%)[3] |
| Chemical and physical data | |
| Formula | C8H5F3N2OS |
| Molar mass | 234.20 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Riluzole, sold under the brand name Rilutek among others, is a medication used to treat amyotrophic lateral sclerosis.[1] It delays the need for a ventilator or tracheostomy and improves survival by two to three months.[4] It is taken by mouth, either as a tablet or liquid.[5]
Common side effects include weakness and liver problems.[2] Other side effects may include anaphylaxis, interstitial lung disease, and low neutrophils.[1] How it works is unclear, but may be due to a decrease in glutamate in the nervous system.[2]
Riluzole was approved for medical use in the United States in 1995 and Europe in 1996.[1][2] It is available as a generic medication.[5] In the United Kingdom 4 weeks costs the NHS about £23 as of 2021.[5] This amount in the United States is about 33 USD.[6]
Medical use
Amyotrophic lateral sclerosis
Riluzole was approved in the United States for the treatment of ALS by the U.S. Food and Drug Administration (FDA) in 1995.[7] A Cochrane Library review states a 9% gain in the probability of surviving one year.[4]
Dosage
It is taken at a dose of 50 mg twice per day.[2]
Side effects
- Very common (>10% frequency):[8] nausea; weakness; decreased lung function
- Common (1–10% frequency):[9] headache; dizziness; drowsiness; vomiting; abdominal pain; increased aminotransferases
- Uncommon (0.1-1% frequency):[9] pancreatitis; interstitial lung disease
- Rare (<0.1% frequency):[9] neutropenia; allergic reaction (including angiooedema, anaphylactoid reaction)
Overdose
Symptoms of overdose include: neurological and psychiatric symptoms, acute toxic encephalopathy with stupor, coma and methemoglobinemia.[3] Severe methemoglobinemia may be rapidly reversible after treatment with methylene blue.[3]
Contraindications
Contraindications for riluzole include: known prior hypersensitivity to riluzole or any of the excipients inside the preparations, liver disease, pregnancy or lactation.[3]
Interactions
CYP1A2 substrates, inhibitors and inducers would probably interact with riluzole, due its dependency on this cytochrome for metabolism.[3]
Mechanism of action
Riluzole preferentially blocks TTX-sensitive sodium channels, which are associated with damaged neurons.[10][11] Riluzole has also been reported to directly inhibit the kainate and NMDA receptors.[12] The drug has also been shown to postsynaptically potentiate GABAA receptors via an allosteric binding site.[13] However, the action of riluzole on glutamate receptors has been controversial, as no binding of the drug to any known sites has been shown for them.[14][15] In addition, as its antiglutamatergic action is still detectable in the presence of sodium channel blockers, it is also uncertain whether or not it acts via this way. Rather, its ability to stimulate glutamate uptake seems to mediate many of its effects.[16][17] In addition to its role in accelerating glutamate clearance from the synapse, riluzole may also prevent glutamate release from presynaptic terminals.[18] Since CK1δ plays a key role in TDP-43 proteinopathy, a pathological hallmark of ALS, this could help to better decipher drug mechanism of action.
Society and culture
Legal status
Riluzole was approved for medical use in the European Union in October 1996.[19]
Synthesis
Riluzole can be prepared beginning with the reaction of 4-(trifluoromethoxy)aniline with potassium thiocyanate followed by reaction with bromine, forming the thiazole ring.[20][21][22]
 Riluzole synthesis
Riluzole synthesis
Research
A number of case studies have indicated that riluzole may have use in mood and anxiety disorders.[23]
A reformulation of riluzole that originated at Yale University and is known by the code name BHV-0223[24] is under development for the treatment of generalized anxiety disorder and mood disorders by Biohaven Pharmaceuticals.[25]
Riluzole, which is neuroprotective and a glutamate modulator could be used for psychiatric problems though it failed in trials of Huntington's disease and Parkinson's disease.[26]
See also
References
- 1 2 3 4 "Riluzole Monograph for Professionals". Drugs.com. Archived from the original on August 10, 2020. Retrieved October 17, 2021.
- 1 2 3 4 5 "Rilutek". Archived from the original on December 27, 2020. Retrieved October 17, 2021.
- 1 2 3 4 5 6 7 8 9 "PRODUCT INFORMATION RILUTEK® (riluzole) Tablets" (PDF). TGA eBusiness Services. sanofi-aventis australia pty ltd. January 6, 2009. Archived from the original on March 17, 2018. Retrieved February 18, 2014.
- 1 2 Miller, RG; Mitchell, JD; Moore, DH (March 14, 2012). "Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND)". The Cochrane Database of Systematic Reviews. 3 (3): CD001447. doi:10.1002/14651858.CD001447.pub3. PMC 7055506. PMID 22419278.
- 1 2 3 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 1186. ISBN 978-0-85711-369-6.
- ↑ "Riluzole Prices and Riluzole Coupons - GoodRx". GoodRx. Retrieved October 17, 2021.
- ↑ "Riluzole". Archived from the original on July 2, 2019. Retrieved December 24, 2020.
- ↑ "Rilutek (riluzole) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on May 4, 2018. Retrieved February 18, 2014.
- 1 2 3 Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- ↑ Song, JH; Huang, CS; Nagata, K; Yeh, JZ; Narahashi, T (August 1997). "Differential action of riluzole on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels" (PDF). The Journal of Pharmacology and Experimental Therapeutics. 282 (2): 707–14. PMID 9262334. Archived from the original on August 29, 2021. Retrieved December 24, 2020.
- ↑ Bellingham, MC (February 2011). "A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade?". CNS Neuroscience & Therapeutics. 17 (1): 4–31. doi:10.1111/j.1755-5949.2009.00116.x. PMC 6493865. PMID 20236142.
- ↑ Debono MW, Le Guern J, Canton T, Doble A, Pradier L (April 1993). "Inhibition by riluzole of electrophysiological responses mediated by rat kainate and NMDA receptors expressed in Xenopus oocytes". Eur. J. Pharmacol. 235 (2–3): 283–9. doi:10.1016/0014-2999(93)90147-a. PMID 7685290.
- ↑ He, Y.; Benz, A.; Fu, T.; Wang, M.; Covey, D.F.; Zorumski, C.F.; Mennerick, S. (2002). "Neuroprotective agent riluzole potentiates postsynaptic GABAA receptor function". Neuropharmacology. 42 (2): 199–209. doi:10.1016/s0028-3908(01)00175-7. PMID 11804616. S2CID 24194421.
- ↑ Wokke, J (September 21, 1996). "Riluzole". Lancet. 348 (9030): 795–9. doi:10.1016/S0140-6736(96)03181-9. PMID 8813989. S2CID 208788906.
- ↑ Kretschmer BD, Kratzer U, Schmidt WJ (August 1998). "Riluzole, a glutamate release inhibitor, and motor behavior". Naunyn Schmiedebergs Arch. Pharmacol. 358 (2): 181–90. doi:10.1007/pl00005241. PMID 9750003. S2CID 5887788.
- ↑ Azbill, RD; Mu, X; Springer, JE (July 2000). "Riluzole increases high-affinity glutamate uptake in rat spinal cord synaptosomes". Brain Res. 871 (2): 175–80. doi:10.1016/S0006-8993(00)02430-6. PMID 10899284. S2CID 23849619.
- ↑ Dunlop, J; Beal McIlvain, H; She, Y; Howland, DS (March 1, 2003). "Impaired spinal cord glutamate transport capacity and reduced sensitivity to riluzole in a transgenic superoxide dismutase mutant rat model of amyotrophic lateral sclerosis". J. Neurosci. 23 (5): 1688–96. doi:10.1523/JNEUROSCI.23-05-01688.2003. PMC 6741992. PMID 12629173.
- ↑ Wang, S.-J (January 2004). "Mechanisms underlying the riluzole inhibition of glutamate release from rat cerebral cortex nerve terminals (synaptosomes)". Neuroscience. 125 (1): 191–201. doi:10.1016/j.neuroscience.2004.01.019. PMID 15051158. S2CID 35667296.
- ↑ "Rilutek EPAR". European Medicines Agency (EMA). Archived from the original on December 27, 2020. Retrieved October 1, 2020.
- ↑ L. M. Yagupol'skii, L. Z. Gandel'sman, Zh. Obshch. Khim. 33, 2301 (1963), C.A. 60, 692a (1964).
- ↑ J. Mizoule, EP 50551; idem, U.S. Patent 4,370,338 (1982, 1983 both to Pharmindustrie).
- ↑ U.S. Patent 4,826,860
- ↑ Grant, P; Song, JY; Swedo, SE (2010). "Review of the use of the glutamate antagonist riluzole in psychiatric disorders and a description of recent use in childhood obsessive-compulsive disorder". J Child Adolesc Psychopharmacol. 20 (4): 309–15. doi:10.1089/cap.2010.0009. PMC 2958461. PMID 20807069.
- ↑ "BHV 0223 – AdisInsight". Adisinsight.springer.com. Archived from the original on May 9, 2021. Retrieved May 20, 2016.
- ↑ Harris, Elaine (2015). "Industry update: the latest developments in therapeutic delivery". Therapeutic Delivery. 6 (6): 647–652. doi:10.4155/tde.15.44. ISSN 2041-5990.
- ↑ Zarate CA, Manji HK (September 2008). "Riluzole in psychiatry: a systematic review of the literature". Expert Opin Drug Metab Toxicol. 4 (9): 1223–34. doi:10.1517/17425255.4.9.1223. PMC 2587133. PMID 18721116.
External links
| External sites: |
|
|---|---|
| Identifiers: |