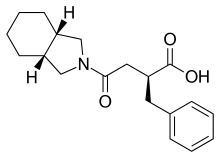
Mitiglinide
 | |
| Clinical data | |
|---|---|
| Trade names | Glufast |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H25NO3 |
| Molar mass | 315.413 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Mitiglinide (INN,[1] trade name Glufast) is a drug for the treatment of type 2 diabetes.[2]
Mitiglinide belongs to the meglitinide (glinide) class of blood glucose-lowering drugs and is currently co-marketed in Japan by Kissei and Takeda. The North America rights to mitiglinide are held by Elixir Pharmaceuticals. Mitiglinide has not yet gained FDA approval.
Pharmacology
Mitiglinide is thought to stimulate insulin secretion by closing the ATP-sensitive potassium KATP channels in pancreatic β cells.
Dosage
Mitiglinide is delivered in tablet form.
References
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary names (Rec. INN): List 40" (PDF). World Health Organization. p. 187. Retrieved 10 November 2016.
- ↑ Malaisse WJ (October 2008). "Mitiglinide: a rapid- and short-acting non-sulfonylurea insulinotropic agent for the treatment of type 2 diabetic patients". Expert Opinion on Pharmacotherapy. 9 (15): 2691–8. doi:10.1517/14656566.9.15.2691. PMID 18803455.
External links
- Elixir Pharmaceuticals — website of the U.S. rights holder for mitiglinide.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.