Imeglimin
 | |
| Clinical data | |
|---|---|
| Trade names | Twymeeg |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
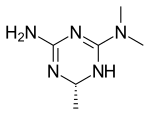
| Formula | C6H13N5 |
| Molar mass | 155.205 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Imeglimin (brand name Twymeeg) is an oral anti-diabetic medication.[1][2] It was approved for use in Japan in June 2021.[3]
It is an oxidative phosphorylation blocker that acts to inhibit hepatic gluconeogenesis, increase muscle glucose uptake, and restore normal insulin secretion. It is the first approved drug of this class of anti-diabetic medication.
References
- ↑ Vuylsteke V, Chastain LM, Maggu GA, Brown C (September 2015). "Imeglimin: A Potential New Multi-Target Drug for Type 2 Diabetes". Drugs in R&D. 15 (3): 227–32. doi:10.1007/s40268-015-0099-3. PMC 4561051. PMID 26254210.
- ↑ Dubourg J, Fouqueray P, Thang C, Grouin JM, Ueki K (April 2021). "Efficacy and Safety of Imeglimin Monotherapy Versus Placebo in Japanese Patients With Type 2 Diabetes (TIMES 1): A Double-Blind, Randomized, Placebo-Controlled, Parallel-Group, Multicenter Phase 3 Trial". Diabetes Care. 44 (4): 952–959. doi:10.2337/dc20-0763. PMID 33574125.
- ↑ Poxel SA (June 23, 2021). "Poxel and Sumitomo Dainippon Pharma Announce the Approval of TWYMEEG® (Imeglimin hydrochloride) for the Treatment of Type 2 Diabetes in Japan" (Press release).
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.