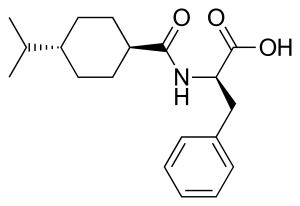
Nateglinide
 | |
| Names | |
|---|---|
| Trade names | Starlix |
IUPAC name
| |
| Clinical data | |
| Drug class | Meglitinide[1] |
| Main uses | Type 2 diabetes[2] |
| Side effects | Dizziness, diarrhea, bronchitis, low blood sugar[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | By mouth |
| Typical dose | 60 to 180 mg TID[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699057 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Protein binding | 98% |
| Elimination half-life | 1.5 hours |
| Chemical and physical data | |
| Formula | C19H27NO3 |
| Molar mass | 317.429 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Nateglinide, sold under the brand name Starlix, is a medication used to treat type 2 diabetes.[2] It is used together with diet and exercise.[2] It is not a first line treatment, though may be added to metformin.[2][3] It is taken by mouth.[2]
Common side effects include dizziness, diarrhea, bronchitis, and low blood sugar.[2] Use is not recommended in those with significant liver problems.[3] Safety in pregnancy is not clear.[2] It belongs to the meglitinide class and works by stimulating the release of insulin.[1]
Nateglinide was approved for medical use in the United States in 2000 and Europe in 2001.[2][3] In the United States it costs about 30 USD per month as of 2021.[4] It is not available in the United Kingdom as of 2021.[1]
Medical uses
Dosage
It is generally take at a dose of 60 to 180 mg half an hour before meals three times per day.[1]
Nateglinide is delivered in 60 mg & 120 mg tablet form.
Contraindications
Nateglinide is contraindicated in patients who:
- have known hypersensitivity to the compound or any ingredient in the formulation.
- are affected with type 1 (namely insulin-dependent) diabetes mellitus.
- are in diabetic ketoacidosis.
Pharmacology
Nateglinide lowers blood glucose by stimulating the release of insulin from the pancreas. It achieves this by closing ATP-dependent potassium channels in the membrane of the β cells. This depolarizes the β cells and causes voltage-gated calcium channels to open. The resulting calcium influx induces fusion of insulin-containing vesicles with the cell membrane, and insulin secretion occurs.
See also
References
- 1 2 3 4 5 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 742. ISBN 978-0857114105.
- 1 2 3 4 5 6 7 8 9 "Nateglinide Monograph for Professionals". Drugs.com. Archived from the original on 2 September 2019. Retrieved 12 November 2021.
- 1 2 3 "Starlix". Archived from the original on 12 November 2020. Retrieved 12 November 2021.
- ↑ "Nateglinide Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 25 April 2020. Retrieved 12 November 2021.
External links
| Identifiers: |
|---|