SGLT2 inhibitor
| SGLT2 inhibitor | |
|---|---|
| Drug class | |
| Names | |
| Other names | Gliflozins |
| Clinical data | |
| Uses | Type 2 diabetes |
SGLT2 inhibitors, also called gliflozins, are a class of medications used to treat type II diabetes mellitus (T2DM). Apart from blood sugar control, they provide cardiovascular benefit in T2DM.[1][2] Several medications have been approved.[3] Canagliflozin, a member of the class, enhances blood sugar control, reduces body weight, and systolic and diastolic blood pressure.[4]
They act on the nephron; unlike SGLT1 inhibitors that modulate sodium/glucose channels in the intestinal mucosa. They inhibit reabsorption of glucose in the kidney and therefore lower blood sugar.[5] They act by inhibiting sodium-glucose transport protein 2 (SGLT2).
In the United States they cost between 400 and 700 USD per month as of 2022.[6] In Canada they are about 90 CAD per month as of 2022.[7]
Medical uses
Type 2 diabetes
The gliflozins are used to treat type 2 diabetes but are most often used as second- or third-line agents as there are other medications that have much longer safety record and are less expensive.[8]
Gliflozins may be a good option for people who are failing with metformin alone, especially if reducing weight is part of the underlying treatment.[9][2] They are often used in combination therapy, for example the dual therapy metformin plus gliflozin and the triple therapy metformin, sulfonylurea and gliflozin.[8]
A review comparing SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors found that use of SGLT2 inhibitors was associated with a 20% reduction in death compared with no treatment.[10]
Heart failure
In heart failure with preserved or mildly decreased ejection fraction SGLT2 inhibitors decrease hospitalization but do not change the risk of death.[11] In heart failure with reduced ejection fraction it improves life expectancy, reduces hospitalization, and improves quality of life.[12]
Side effects
Genital infections seem to be the most common side effect. Fungal infections, urinary tract infections and osmotic diuresis were higher.
In 2015, the FDA issued a warning of an increased risk of diabetic ketoacidosis (DKA).[13] By reducing glucose blood circulation, gliflozins cause less stimulation of endogenous insulin secretion or lower dose of exogenous insulin that results in diabetic ketoacidosis. They can specifically cause euglycemic DKA (euDKA, DKA where the blood sugar is not elevated) because of the renal tubular absorption of ketone bodies.[14] A particularly high risk period for ketoacidosis is the perioperative period. SGLT2 inhibitors may need to be discontinued before surgery, and only recommended when someone is not unwell, is adequately hydrated and able to consume a regular diet.[15]
In 2015, the FDA issued a warning related to canagliflozin and canagliflozin/metformin due to decreased bone mineral density and therefore increased risk of bone fractures. Using gliflozins in combination therapy with metformin can lower the risk of hypoglycemia compared to other T2DM such as sulfonylureas and insulin.[13]
Increased risk of lower limb amputation is associated with canagliflozin but further data is needed to confirm this risk associated with different gliflozins.[16] A European Medicines Agency review concluded that there is an increased risk of lower limb amputation (mostly affecting the toes) in people taking canagliflozin, dapagliflozin and empagliflozin.[17]
In 2018, the FDA issued a warning of an increased risk of Fournier gangrene with SGLT2 inhibitors.[18] The absolute risk is considered very low.[19]
To lessen the risk of developing ketoacidosis (a serious condition in which the body produces high levels of blood acids called ketones) after surgery, the FDA has approved changes to the prescribing information for SGLT2 inhibitor diabetes medicines to recommend they be stopped temporarily before scheduled surgery. Canagliflozin, dapagliflozin, and empagliflozin should each be stopped at least three days before, and ertugliflozin should be stopped at least four days before scheduled surgery.[20]
Symptoms of ketoacidosis include nausea, vomiting, abdominal pain, tiredness, and trouble breathing.[20]
Interactions
Interactions are important for SGLT2 inhibitors because most T2DM patients are taking many other medications. Gliflozins appear to increase the diuretic effect of thiazides, loop diuretics and related diuretics and may increase the risk of dehydration and hypotension.[21] It is important to adjust the dose of antidiabetics if the treatment is combination therapy to avoid hypoglycemia. For example, interactions with sulfonylureas have led to severe hypoglycemia presumably due to cytochrome P450.[22]
A study has shown that it is safe to consume dapagliflozin along with pioglitazone, metformin, glimepiride, or sitagliptin and dose adjustment is unnecessary for either medication.[23] It is unlikely that food intake has clinical meaningful impact on the efficacy of dapagliflozin, therefore it can be administered without regard to meals.[23][24]
Types
Members of the gliflozin class:
- Canagliflozin was the first SGLT2 inhibitor to be approved for use in the United States. It was approved in March 2013, under the brand name Invokana and it was also marketed throughout the EU under the same name.[25][26]
- Dapagliflozin is the first SGLT2 inhibitor approved anywhere in the world by the EU in 2012.[27] It was approved for use in the United States under the brand name Farxiga by the Food and Drug Administration in January 2014.[28] the first oral treatment in combination with insulin to treat type 1 diabetes mellitus in UK and EU.
- Empagliflozin, approved in the United States in August 2014, under the brand name Jardiance by Boehringer Ingelheim.[29] Of the gliflozins, empagliflozin and tofogliflozin have the highest specificity for SGLT2 inhibition.[5] It is the only oral medicine for type 2 diabetes that has been shown to reduce the risk of cardiovascular death.[30]
- Ertugliflozin was approved in the United States under the brand name Steglatro in December 2017.[31]
- Ipragliflozin, produced by the Japanese company Astellas Pharma Inc. under the brand name Suglat, approved in Japan January 2014.[32][33]
- Luseogliflozin was approved in Japan March 2014 under the brand name Lusefi and was developed by Taisho Pharmaceutical.[34]
- Remogliflozin etabonate was commercially launched first in India by Glenmark in May 2019.
- Sergliflozin etabonate discontinued after Phase II trials.[35]
- Sotagliflozin is a dual SGLT1/SGLT2 inhibitor in phase III trials under the brand name Zynquista. Developed by Lexicon pharmaceuticals. It was planned to be the first oral treatment in combination with insulin to treat type 1 diabetes mellitus.[36] The Food and Drug Administration refused its approval for use in combination with insulin for the treatment of type 1 diabetes.[37][38]
- Tofogliflozin was approved in Japan in March 2014, under the brand names Apleway and Deberza developed by Sanofi and Kowa Pharmaceutical.[39]
Mechanism of action
SGLT-2 inhibitors improve cardio-renal function in patients with type 2 diabetes mellitus, emphasizing the impacts in improving neural tone.[40] Sodium glucose cotransporters (SGLTs) are proteins that occur primarily in the kidneys and play an important role in maintaining glucose balance in the blood.[41] SGLT1 and SGLT2 are the two most known SGLTs of this family. SGLT2 is the major transport protein and promotes reabsorption from the glomerular filtration glucose back into circulation and is responsible for approximately 90% of the kidney's glucose reabsorption.[5] SGLT2 is mainly expressed in the kidneys on the epithelial cells lining the first segment of the proximal convoluted tubule. By inhibiting SGLT2, gliflozins prevent the kidneys' reuptake of glucose from the glomerular filtrate and subsequently lower the glucose level in the blood and promote the excretion of glucose in the urine (glucosuria).[42][43]
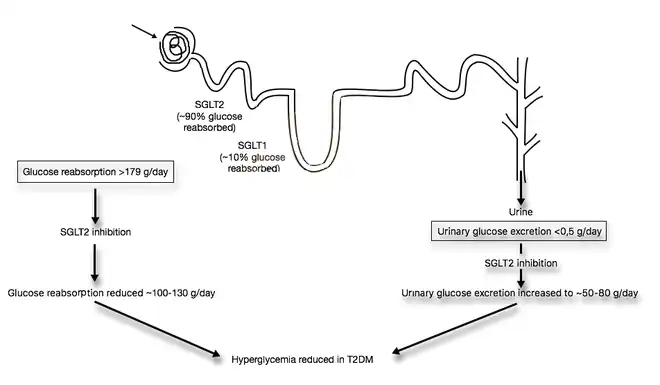
The mechanism of action on a cellular level is not well understood. Work is underway to define this mechanism as a prodiuretic with great promise. However, it has been shown that binding of different sugars to the glucose site affects the orientation of the aglycone in the access vestibule. So when the aglycone binds it affects the entire inhibitor. Together these mechanisms lead to a synergistic interaction. Therefore, variations in the structure of both the sugar and the aglycone are crucial for the pharmacophore of SGLT inhibitors.[44]
Dapagliflozin is an example of an SGLT-2 inhibitor, it is a competitive, highly selective inhibitor of SGLT. It acts via selective and potent inhibition of SGLT-2, and its activity is based on each patient's underlying blood sugar control and kidney function. The results are decreased kidney reabsorption of glucose, glucosuria effect increases with higher level of glucose in the blood circulation. Therefore, dapagliflozin reduces the blood glucose concentration with a mechanism that is independent of insulin secretion and sensitivity, unlike many other antidiabetic medications. Functional pancreatic β-cells are not necessary for the activity of the medication so it is convenient for patients with diminished β-cell function.[42][43]
Sodium and glucose are co-transported by the SGLT-2 protein into the tubular epithelial cells across the brush-border membrane of the proximal convoluted tubule. This happens because of the sodium gradient between the tubule and the cell and therefore provides a secondary active transport of glucose. Glucose is later reabsorbed by passive transfer of endothelial cells into the interstitial glucose transporter protein.[42][43][45]
| SGLT | Expressed in human tissues |
|---|---|
| SGLT1 | Intestine, trachea, kidney, heart, brain, testis, prostate |
| SGLT2 | Kidney, brain, liver, thyroid, muscle, heart |
Ratios of activity between SGLT1 and SGLT2 may be helpful in defining expression.
Pharmacology
The elimination half-life, bioavailability, protein binding, the blood concentration Cmax at time tmax, and other pharmacokinetic parameters of various medications of this class are present in table 2. These medications are excreted in the urine as inactive metabolites.[45][46][47][48]
| Name of drug | Bioavailability | Protein binding | tmax (hours) | t1/2 (hours) | Cmax | SGLT2 selectivity over SGLT1 |
|---|---|---|---|---|---|---|
| Canagliflozin | 65% (300 mg dose) | 99% | 1–2 | 10.6 (100 mg dose); 13.1 (300 mg dose) | 1096 ng/mL (100 mg dose); 3480 ng/mL (300 mg dose) | 250 fold |
| Dapagliflozin | 78% | 91% | 1–1.5 | 12.9 | 79.6 ng/mL (5 mg dose); 165.0 ng/mL (10 mg dose) | 1200 fold |
| Empagliflozin | 90–97% (mice); 89% (dogs); 31% (rats) | 86.20% | 1.5 | 13.2 (10 mg dose); 13.3h (25 mg dose) | 259nmol/L (10 mg dose); 687nmol/L (25 mg dose) | 2500 fold |
| Ertugliflozin | 70-90% | 95% | 0.5-1.5 | 11-17 | 268 ng/mL (15 mg dose) | 2000 fold |
| Ipragliflozin (50 mg) | 90% | 96.30% | 1 | 15–16 (50 mg dose) | 975 ng/mL | 360 fold |
| Luseogliflozin | 35.3% (male rats); 58.2% (female rats); 92.7% (male dogs) | 96.0–96.3% | 0.625±0.354 | 9.24±0.928 | 119±27.0 ng/mL | 1650 fold |
| Tofogliflozin (10 mg) | 97.50% | 83% | 0.75 | 6.8 | 489 ng/mL | 2900 fold |
- Cmax: Maximum serum concentration that drug achieves in body after the drug has been and administrated
- tmax: Time to achieve maximum plasma concentration
- t1/2: Biological half-life
In studies that were made on healthy people and people with type II diabetes mellitus, who were given dapagliflozin in either single ascending dose (SAD) or multiple ascending dose (MAD) showed results that confirmed a pharmacokinetic profile of the medication. With dose-dependent concentrations the half-life is about 12–13 hours, Tmax 1–2 hours and it is protein-bound, so the medication has a rapid absorption and minimal excretion by the kidney.[23]
Dapagliflozin disposition is not evidently affected by BMI or body weight, therefore the pharmacokinetic findings are expected to be applicable to patients with a higher BMI. Dapagliflozin resulted in dose-dependent increases excretions in urinary glucose, up to 47g/d following single-dose administration, which can be expected from its mechanism of action, dapagliflozin.[24]
In some long term clinical studies that have been made on dapagliflozin, dapagliflozin was associated with a decrease in body weight which was statistically superior compared to placebo or other active comparators. It is primarily associated with caloric rather than fluid loss.[24][45]
Structure-activity relationship
The structure-activity relationship (SAR) of gliflozins is not fully understood.
The most common gliflozins are dapagliflozin, empagliflozin and canagliflozin. The differences in the structures is relatively small. The general structure includes a glucose sugar with an aromatic group in the β-position at the anomeric carbon. In addition to the glucose sugar moiety and the β-isomeric aryl substituent the aryl group is composed of a diarylmethylene structure.
The synthesis of Gliflozins involves three general steps. The first one is the construction of the aryl substituent, the next one is the introduction of the aryl moiety onto the sugar or glucosylation of the aryl substituent and the last one the deprotection and modification of the arylated anomeric center of the sugar.[50]
Phlorizin was the first type of gliflozin and it was non-selective against SGLT2/SGLT1. It is a natural O-aryl glycoside composed of a d-glucose and an aromatic ketone. However Phlorizin is very unstable, it is rapidly degraded by glucosidases in the small intestines, so it can not be used as an oral administration medication to treat diabetes. Structural modifications have been made to overcome this instability problem. The most efficient way was to conjugate aryl moiety with glucose moiety since C-glucosides are more stable in the small intestines than O-glucoside derivatives (C-C bond instead of C-O-C bond).[51]
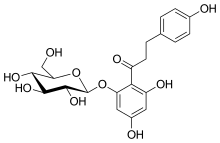
In the sugar analogues of dapagliflozin, the β-C series are more active than α-C series so it is critical that the β-configuration is at C-1 for the inhibitory activity.[52] Both dapagliflozin and empagliflozin contain a chlorine (Cl) atom in their chemical structure. Cl is a halogen and it has a high electronegativity. This electronegativity withdraws electrons off the bonds and therefore it reduces the metabolism. The Cl atom also reduces the IC50 value of the medication so the medication has better activity. The carbon-fluorine bond (C-F) has also has a veryl low electron density.[53]
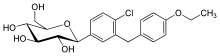
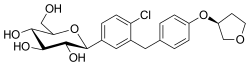
For example in the chemical structure of canagliflozin a fluorine atom is connected to an aromatic ring then the compound is more stable and the metabolism of the compound is reduced. Empagliflozin contains a tetrahydrofuran ring but not canagliflozin nor dapagliflozin.[54]
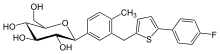
In the development of gliflozins the distal ring contains a thiophene ring instead of an aromatic ring. However the final chemical structures of the marketing gliflozins does not contain this thiophene ring.[55]
Alternative actions
Gliflozins have been posited to exhibit protective effects on the heart, liver, kidneys, anti‐hyperlipidemic, anti‐atherosclerotic, anti‐obesity, anti‐neoplastic effects in in vitro, pre‐clinical, and clinical studies. Pleiotropic effects of this class have been attributed to a variety of its pharmacodynamic actions such as natriuresis, hemoconcentration, deactivation of renin-angiotensin-aldosterone system, ketone body formation, alterations in energy homeostasis, glycosuria, lipolysis, anti‐inflammatory, and antioxidative actions.[56][2]
History
Phlorizin is a molecule with SGLT inhibiting properties, and served an important role in the development of the gliflozin class of medications.
References
- ↑ Usman, Muhammad Shariq; Siddiqi, Tariq Jamal; Memon, Muhammad Mustafa; Khan, Muhammad Shahzeb; Rawasia, Wasiq Faraz; Talha Ayub, Muhammad; Sreenivasan, Jayakumar; Golzar, Yasmeen (2018). "Sodium-glucose co-transporter 2 inhibitors and cardiovascular outcomes: A systematic review and meta-analysis". European Journal of Preventive Cardiology. 25 (5): 495–502. doi:10.1177/2047487318755531. PMID 29372664. S2CID 3557967.
- 1 2 3 Bonora BM, Avogaro A, Fadini GP (2020). "Extraglycemic Effects of SGLT2 Inhibitors: A Review of the Evidence". Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 13: 161–174. doi:10.2147/DMSO.S233538. PMC 6982447. PMID 32021362.
- ↑ Scheen, André J. (2014). "Pharmacodynamics, Efficacy and Safety of Sodium–Glucose Co-Transporter Type 2 (SGLT2) Inhibitors for the Treatment of Type 2 Diabetes Mellitus". Drugs. 75 (1): 33–59. doi:10.1007/s40265-014-0337-y. PMID 25488697. S2CID 9350259.
- ↑ Haas, B.; Eckstein, N.; Pfeifer, V.; Mayer, P.; Hass, M D S. (2014). "Efficacy, safety and regulatory status of SGLT2 inhibitors: Focus on canagliflozin". Nutrition & Diabetes. 4 (11): e143. doi:10.1038/nutd.2014.40. PMC 4259905. PMID 25365416.
- 1 2 3 Shubrook, Jay; Baradar-Bokaie, Babak; Adkins, Sarah (2015). "Empagliflozin in the treatment of type 2 diabetes: Evidence to date". Drug Design, Development and Therapy. 9: 5793–803. doi:10.2147/DDDT.S69926. PMC 4634822. PMID 26586935.
- ↑ American Diabetes Association Professional Practice Committee (January 2022). "9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2022". Diabetes Care. 45 (Suppl 1): S125–S143. doi:10.2337/dc22-S009. PMID 34964831. S2CID 245538347.
- ↑ Ton, Joey (19 July 2022). "#319 Should a 'flozin' be chosen? Part 2: SGLT2 inhibitors in patients with chronic kidney disease". CFPCLearn. Archived from the original on 2 July 2023. Retrieved 14 June 2023.
- 1 2 Clar, Christine; Gill, James Alexander; Court, Rachel; Waugh, Norman (2012). "Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes". BMJ Open. 2 (5): e001007. doi:10.1136/bmjopen-2012-001007. PMC 3488745. PMID 23087012.
- ↑ Nauck, Michael (2014). "Update on developments with SGLT2 inhibitors in the management of type 2 diabetes". Drug Design, Development and Therapy. 8: 1335–80. doi:10.2147/DDDT.S50773. PMC 4166348. PMID 25246775.
- ↑ Zheng, Sean L.; Roddick, Alistair J.; Aghar-Jaffar, Rochan; Shun-Shin, Matthew J.; Francis, Darrel; Oliver, Nick; Meeran, Karim (2018). "Association Between Use of Sodium-Glucose Cotransporter 2 Inhibitors, Glucagon-like Peptide 1 Agonists, and Dipeptidyl Peptidase 4 Inhibitors with All-Cause Mortality in Patients with Type 2 Diabetes". JAMA. 319 (15): 1580–1591. doi:10.1001/jama.2018.3024. PMC 5933330. PMID 29677303.
- ↑ Ton, Joey (7 March 2022). "#310 Medications for Heart Failure with Preserved or Mildly-Reduced Ejection Fraction: Heart Failure or Heart Success?". CFPCLearn. Archived from the original on 30 June 2023. Retrieved 14 June 2023.
- ↑ Ton, Joey (11 January 2022). "#306 Top 5 Tools for Practice of 2021". CFPCLearn. Archived from the original on 1 July 2023. Retrieved 14 June 2023.
- 1 2 Hsia, Daniel S.; Grove, Owen; Cefalu, William T. (2016). "An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus". Current Opinion in Endocrinology, Diabetes and Obesity. 24 (1): 73–79. doi:10.1097/MED.0000000000000311. PMC 6028052. PMID 27898586.
- ↑ Isaacs, Michelle; Tonks, Katherine T.; Greenfield, Jerry R. (2017). "Euglycaemic diabetic ketoacidosis in patients using sodium-glucose co-transporter 2 inhibitors". Internal Medicine Journal. 47 (6): 701–704. doi:10.1111/imj.13442. PMID 28580740. S2CID 4091595.
- ↑ Milder, D. A.; Milder, T. Y.; Kam, P. C. A. (August 2018). "Sodium-glucose co-transporter type-2 inhibitors: pharmacology and peri-operative considerations". Anaesthesia. 73 (8): 1008–1018. doi:10.1111/anae.14251. PMID 29529345.
- ↑ Khouri, Charles; Cracowski, Jean-Luc; Roustit, Matthieu (2018). "SGLT-2 inhibitors and the risk of lower-limb amputation: Is this a class effect?". Diabetes, Obesity and Metabolism. 20 (6): 1531–1534. doi:10.1111/dom.13255. PMID 29430814. S2CID 3873882.
- ↑ "SGLT2 inhibitors: information on potential risk of toe amputation to be included in prescribing information". European Medicines Agency. 4 May 2017. Archived from the original on 22 July 2021. Retrieved 9 June 2021.
- ↑ "FDA warns about rare occurrences of a serious infection of the genital area with SGLT2 inhibitors for diabetes". www.fda.gov. Center for Drug Evaluation and Research. 7 September 2018. p. Drug Safety and Availability. Archived from the original on 7 February 2019. Retrieved 16 April 2019.
- ↑ Bardia, Amit; Wai, Mabel; Fontes, Manuel L. (February 2019). "Sodium-glucose cotransporter-2 inhibitors: an overview and perioperative implications". Current Opinion in Anesthesiology. 32 (1): 80–85. doi:10.1097/ACO.0000000000000674. PMID 30531609. S2CID 54471240. Archived from the original on 2021-11-01. Retrieved 2021-06-09.
- 1 2 "FDA revises labels of SGLT2 inhibitors for diabetes to include warning". U.S. Food and Drug Administration. 19 March 2020. Archived from the original on 7 June 2020. Retrieved 6 June 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ BNF 73. Tavistock Square, London: BMJ Group. March–September 2017.
- ↑ Scheen, André J. (2014). "Drug–Drug Interactions with Sodium-Glucose Cotransporters Type 2 (SGLT2) Inhibitors, New Oral Glucose-Lowering Agents for the Management of Type 2 Diabetes Mellitus". Clinical Pharmacokinetics (Submitted manuscript). 53 (4): 295–304. doi:10.1007/s40262-013-0128-8. hdl:2268/164207. PMID 24420910. S2CID 5228432. Archived from the original on 2021-11-01. Retrieved 2021-06-09.
- 1 2 3 Bhartia, Mithun; Tahrani, Abd A.; Barnett, Anthony H. (2011). "SGLT-2 Inhibitors in Development for Type 2 Diabetes Treatment". The Review of Diabetic Studies. 8 (3): 348–354. doi:10.1900/RDS.2011.8.348. PMC 3280669. PMID 22262072.
- 1 2 3 Yang, Li; Li, Haiyan; Li, Hongmei; Bui, Anh; Chang, Ming; Liu, Xiaoni; Kasichayanula, Sreeneeranj; Griffen, Steven C.; Lacreta, Frank P.; Boulton, David W. (2013). "Pharmacokinetic and Pharmacodynamic Properties of Single- and Multiple-Dose of Dapagliflozin, a Selective Inhibitor of SGLT2, in Healthy Chinese Subjects". Clinical Therapeutics. 35 (8): 1211–1222.e2. doi:10.1016/J.Clinthera.2013.06.017. PMID 23910664.
- ↑ "Drug Approval Package: Invokana (canagliflozin) Tablets NDA #204042". U.S. Food and Drug Administration (FDA). 24 December 1999. Archived from the original on 3 April 2021. Retrieved 5 May 2020.
- ↑ "Invokana EPAR". European Medicines Agency (EMA). Archived from the original on 2 October 2018. Retrieved 1 October 2018.
- ↑ "Forxiga EPAR". European Medicines Agency (EMA). Archived from the original on 17 February 2020. Retrieved 17 February 2020.
- ↑ "Drug Approval Package: Farxiga (dapagliflozin) Tablets NDA #202293". U.S. Food and Drug Administration (FDA). 24 December 1999. Archived from the original on 19 September 2020. Retrieved 5 May 2020.
- ↑ "FDA approves Jardiance (empagliflozin) tablets for adults with type 2 diabetes". Boehringer Ingelheim / Eli Lilly and Company. 1 August 2014. Archived from the original on 5 November 2014. Retrieved 5 November 2014.
- ↑ "FDA Advisory Committee recommends approval of Jardiance (empagliflozin) for cardiovascular indication in 12-11 vote". Yahoo! Finance. 28 June 2016. Archived from the original on 15 September 2016. Retrieved 10 August 2016.
- ↑ "Steglatro (ertugliflozin), Steglujan (ertugliflozin and sitagliptin), Segluromet (ertugliflozin and metformin hydrochloride) Tablets". U.S. Food and Drug Administration (FDA). 5 March 2018. Archived from the original on 24 October 2020. Retrieved 6 May 2020.
- ↑ "Approval of Suglat® Tablets, a Selective SGLT2 Inhibitor for Treatment of Type 2 Diabetes, in Japan". January 17, 2014. Archived from the original on September 23, 2015. Retrieved June 9, 2021.
- ↑ Poole, Raewyn M.; Dungo, Rosselle T. (26 March 2014). "Ipragliflozin: First Global Approval". Drugs. 74 (5): 611–617. doi:10.1007/s40265-014-0204-x. PMID 24668021. S2CID 19837125.
- ↑ Markham, Anthony; Elkinson, Shelley (2014). "Luseogliflozin: First Global Approval". Drugs. 74 (8): 945–950. doi:10.1007/s40265-014-0230-8. PMID 24848756. S2CID 1770988.
- ↑ Da Silva, Paula Nogueira; Da Conceição, Raissa Alves; Do Couto Maia, Rodolfo; De Castro Barbosa, Maria Leticia (2018). "Sodium–glucose cotransporter 2 (SGLT-2) inhibitors: A new antidiabetic drug class". MedChemComm. 9 (8): 1273–1281. doi:10.1039/c8md00183a. PMC 6096352. PMID 30151080.
- ↑ Clinical trial number NCT02531035 for "A Phase 3 Study to Evaluate the Safety of Sotagliflozin in Patients With Type 1 Diabetes Who Have Inadequate Glycemic Control With Insulin Therapy Alone (inTandem3)" at ClinicalTrials.gov
- ↑ "Sotagliflozin as an Adjunct to Insulin for Type 1 Diabetes - FDA" (PDF). Archived (PDF) from the original on 2019-04-26. Retrieved 2021-06-09.
- ↑ Sanofi (17 January 2019). "Sanofi: FDA advisory committee votes on Zynquista(TM) (sotagliflozin) as treatment for adults with type 1 diabetes". GlobeNewswire News Room. Archived from the original on 1 August 2019. Retrieved 9 June 2021.
- ↑ Poole, Raewyn M.; Prossler, Jennifer E. (2014). "Tofogliflozin: First Global Approval". Drugs. 74 (8): 939–944. doi:10.1007/s40265-014-0229-1. PMID 24848755. S2CID 37021884.
- ↑ Nashawi, Mouhamed; Sheikh, Omar; Battisha, Ayman; Ghali, Abdullah; Chilton, Robert (May 2021). "Neural tone and cardio-renal outcomes in patients with type 2 diabetes mellitus: a review of the literature with a focus on SGLT2 inhibitors". Heart Failure Reviews. 26 (3): 643–652. doi:10.1007/s10741-020-10046-w. ISSN 1573-7322. PMID 33169337. Archived from the original on 2021-05-16. Retrieved 2021-06-09.
- ↑ Chao, E. C. (2014). "SGLT-2 Inhibitors: A New Mechanism for Glycemic Control". Clinical Diabetes. 32 (1): 4–11. doi:10.2337/diaclin.32.1.4. PMC 4521423. PMID 26246672.
- 1 2 3 Anderson, Sarah L.; Marrs, Joel C. (2012). "Dapagliflozin for the Treatment of Type 2 Diabetes". Annals of Pharmacotherapy. 46 (4): 590–598. doi:10.1345/aph.1Q538. PMID 22433611. S2CID 207264502.
- 1 2 3 Li, An-Rong; Zhang, Jian; Greenberg, Joanne; Lee, Taeweon; Liu, Jiwen (2011). "Discovery of non-glucoside SGLT2 inhibitors". Bioorganic & Medicinal Chemistry Letters. 21 (8): 2472–2475. doi:10.1016/j.bmcl.2011.02.056. PMID 21398124.
- ↑ Hummel, Charles S.; Lu, Chuan; Liu, Jie; Ghezzi, Chiari; Hirayama, Bruce A.; Loo, Donald D. F.; Kepe, Vladimir; Barrio, Jorge R.; Wright, Ernest M. (2012). "Structural selectivity of human SGLT inhibitors". American Journal of Physiology. Cell Physiology. 302 (2): C373–C382. doi:10.1152/ajpcell.00328.2011. PMC 3328840. PMID 21940664.
- 1 2 3 Plosker, Greg L. (2012). "Dapagliflozin". Drugs. 72 (17): 2289–2312. doi:10.2165/11209910-000000000-00000. PMID 23170914.
- ↑ "Jardiance". drugs.com. Drugs.com. Archived from the original on 2 November 2014. Retrieved 31 October 2014.
- ↑ "Farxiga". drugs.com. Drugs.com. Archived from the original on 2 November 2014. Retrieved 31 October 2014.
- ↑ "Invokana". drugs.com. Drugs.com. Archived from the original on 2 November 2014. Retrieved 31 October 2014.
- ↑ Madaan, Tushar; Akhtar, Mohd.; Najmi, Abul Kalam (2016). "Sodium glucose Co Transporter 2 (SGLT2) inhibitors: Current status and future perspective". European Journal of Pharmaceutical Sciences. 93: 244–252. doi:10.1016/j.ejps.2016.08.025. PMID 27531551.
- ↑ LARSON, GERALD L. (March–April 2015). "The synthesis of gliflozins". Chimica Oggi - Chemistry Today. 33 (2): 37–40. Archived from the original on 2018-09-30. Retrieved 2021-06-09.
- ↑ Chen, Zeng-Hao; Wang, Ruo-Wen; Qing, Feng-Ling (2012). "Synthesis and biological evaluation of SGLT2 inhibitors: Gem-difluoromethylenated Dapagliflozin analogs". Tetrahedron Letters. 53 (17): 2171–2176. doi:10.1016/j.tetlet.2012.02.062.
- ↑ Ng, Wai-Lung; Li, Ho-Chuen; Lau, Kit-Man; Chan, Anthony K. N.; Lau, Clara Bik-San; Shing, Tony K. M. (17 July 2017). "Concise and Stereodivergent Synthesis of Carbasugars Reveals Unexpected Structure-Activity Relationship (SAR) of SGLT2 Inhibition". Scientific Reports. 7 (1): 5581. Bibcode:2017NatSR...7.5581N. doi:10.1038/s41598-017-05895-9. ISSN 2045-2322. PMC 5514135. PMID 28717146.
- ↑ Ng, Wai-Lung; Li, Ho-Chuen; Lau, Kit-Man; Chan, Anthony K. N.; Lau, Clara Bik-San; Shing, Tony K. M. (2017). "Concise and Stereodivergent Synthesis of Carbasugars Reveals Unexpected Structure-Activity Relationship (SAR) of SGLT2 Inhibition". Scientific Reports. 7 (1): 5581. Bibcode:2017NatSR...7.5581N. doi:10.1038/s41598-017-05895-9. PMC 5514135. PMID 28717146.
- ↑ "7.5: Electron Affinities". Chemistry LibreTexts. 18 November 2014. Archived from the original on 30 September 2018. Retrieved 30 September 2018.
- ↑ Song, Kwang-Seop; Lee, Suk Ho; Kim, Min Ju; Seo, Hee Jeong; Lee, Junwon; Lee, Sung-Han; Jung, Myung Eun; Son, Eun-Jung; Lee, Minwoo; Kim, Jeongmin; Lee, Jinhwa (2010). "Synthesis and SAR of Thiazolylmethylphenyl Glucoside as Novel C-Aryl Glucoside SGLT2 Inhibitors". ACS Medicinal Chemistry Letters. 2 (2): 182–187. doi:10.1021/ml100256c. PMC 4018110. PMID 24900297.
- ↑ Madaan, Tushar; Husain, Ibraheem; Akhtar, Mohamad; Najmi, Abul Kalam (2018). "Exploring novel pharmacotherapeutic applications and repurposing potential of sodium glucose Co Transporter 2 inhibitors". Clinical and Experimental Pharmacology and Physiology. 45 (9): 897–907. doi:10.1111/1440-1681.12963. PMID 29751356.
External links
- "FDA revises label of diabetes drug canagliflozin". U.S. Food and Drug Administration. 15 January 2016. Archived from the original on 28 October 2021. Retrieved 9 June 2021.
- "FDA Drug Safety Communication: FDA confirms increased risk of leg and foot amputations with the diabetes medicine canagliflozin (Invokana, Invokamet, Invokamet XR)". U.S. Food and Drug Administration. 18 May 2016. Archived from the original on 9 March 2021. Retrieved 9 June 2021.
- "FDA Drug Safety Communication: FDA strengthens kidney warnings for diabetes medicines canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR)". U.S. Food and Drug Administration. 17 June 2016. Archived from the original on 10 April 2021. Retrieved 9 June 2021.
- "FDA Drug Safety Communication: Interim clinical trial results find increased risk of leg and foot amputations, mostly affecting the toes, with the diabetes medicine canagliflozin (Invokana, Invokamet); FDA to investigate". U.S. Food and Drug Administration. 9 May 2017. Archived from the original on 2 March 2021. Retrieved 9 June 2021.
- "Warning use metformin in certain patients with reduced kidney function". U.S. Food and Drug Administration. 14 November 2017. Archived from the original on 25 May 2021. Retrieved 9 June 2021.
- "Warning: infection of genital area with SGLT2 inhibitors for diabetes". U.S. Food and Drug Administration. 7 September 2018. Archived from the original on 13 December 2019. Retrieved 9 June 2021.