Fidarestat
 | |
| Names | |
|---|---|
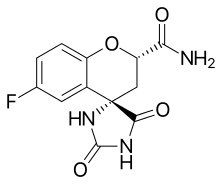
| Preferred IUPAC name
(2S,4S)-6-Fluoro-2′,5′-dioxo-2,3-dihydrospiro[[1]benzopyran-4,4′-imidazolidine]-2-carboxamide | |
| Other names
(2S,4S)-6-fluoro-2',5'-dioxospiro[chroman-4,4'-imidazolidine]-2-carboxamide | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C12H10FN3O4 |
| Molar mass | 279.227 g·mol−1 |
| Melting point | 290–300 °C (554–572 °F; 563–573 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Fidarestat (SNK-860) is an aldose reductase inhibitor under investigation for treatment of diabetic neuropathy.
References
- Aldose reductase inhibitor for treatment of diabetic complications. Prepn (stereo unspec): M. Kurono, et al., EP 193415; eidem, US 4740517 (1986, 1988 both to Sanwa)
- Prepn of isomers: T. Yamaguchi et al., Arzneim.-Forsch. 44, 344 (1994)
- Pharmacological profile: K. Mizuno et al. in Current Concepts of Aldose Reductase and Its Inhibitions, N. Sakamoto et al., Eds. (Elsevier, Amsterdam, 1990) pp 89–96.
- Configuration and crystal structure of complex with aldose reductase: M. Oka et al., J. Med. Chem. 43, 2479 (2000).
- Clinical efficacy in diabetic peripheral neuropathy: N. Hotta et al., Diabetes Care 24, 1776 (2001).
- Clinical suppression of sorbitol accumulation in erythrocytes of diabetic patients: T. Asano et al., J. Diabetes Complications 16, 133 (2002); eidem, ibid. 18, 336 (2004).
- Review of clinical development: N. Giannoukakis, Curr. Opin. Invest. Drugs 4, 1233-1239 (2003).
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.