Evogliptin
 | |
| Clinical data | |
|---|---|
| Trade names | Suganon |
| Other names | DA-1229 |
| Routes of administration | By mouth |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
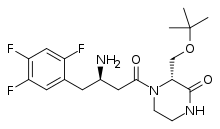
| Formula | C19H26F3N3O3 |
| Molar mass | 401.430 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Evogliptin (INN; trade names Suganon, Evodine) is an antidiabetic drug in the dipeptidyl peptidase-4 (DPP-4) inhibitor or "gliptin" class of drugs.[1] It was developed by the South Korean pharmaceutical company Dong-A ST and is approved for use in South Korea[2] and Russia.[3]
References
- ↑ McCormack PL (November 2015). "Evogliptin: First Global Approval". Drugs. 75 (17): 2045–9. doi:10.1007/s40265-015-0496-5. PMID 26541763.
- ↑ "Dong-A ST's DPP4 inhibitor, SUGANON, got approved for type 2 diabetes in Korea". pipelinereview.com. October 2, 2015.
- ↑ "Evodine (evogliptin) film-coated tablets. Full prescribing information". Russian State Register of Medicines (in Russian).
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.