Teneligliptin
 | |
| Clinical data | |
|---|---|
| Trade names | Tenelia |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
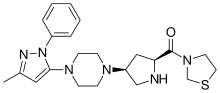
| Formula | C22H30N6OS |
| Molar mass | 426.58 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Teneligliptin (INN; trade name Tenelia) is a pharmaceutical drug for the treatment of type 2 diabetes mellitus. It belongs to the class of anti-diabetic drugs known as dipeptidyl peptidase-4 inhibitors or "gliptins".[1]
Creation
It was created by Mitsubishi Tanabe Pharma and launched in September 2012 by both Mitsubishi Tanabe Pharma and Daiichi Sankyo in Japan.[2]
Licensing and use
Japan/Korea/India/Argentina
It is approved for use in Japan, Argentina, Korea and India.[3]
Pharmacology
Teneligliptin has unique J shaped or anchor locked domain structure because of which it has a potent inhibition of DPP 4 enzyme.
Teneligliptin significantly controls glycemic parameters with safety. No dose adjustment is required in renally impaired patients.[4]
References
- ↑ Kishimoto M (2013). "Teneligliptin: a DPP-4 inhibitor for the treatment of type 2 diabetes". Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 6: 187–95. doi:10.2147/DMSO.S35682. PMC 3650886. PMID 23671395.
- ↑ "TENELIA® 20mg tablets, a Treatment for Type 2 Diabetes Mellitus Approval of Partial Change in Indication to Lift Restrictions in Combination Therapy". Media & Investors. Daiichi Sankyo.
- ↑ Bronson J, Black A, Dhar TG, Ellsworth BA, Merritt JR (2013). Teneligliptin (Antidiabetic), Chapter: To Market, To Market - 2012. Annual Reports in Medicinal Chemistry. Vol. 48. pp. 523–524. doi:10.1016/b978-0-12-417150-3.00028-4. ISBN 9780124171503.
- ↑ Nabeno M, Akahoshi F, Kishida H, Miyaguchi I, Tanaka Y, Ishii S, Kadowaki T (May 2013). "A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site". Biochemical and Biophysical Research Communications. 434 (2): 191–6. doi:10.1016/j.bbrc.2013.03.010. PMID 23501107.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.