Semaglutide
 | |
| Names | |
|---|---|
| Trade names | Ozempic, Rybelsus, Wegovy, others |
| Clinical data | |
| Drug class | GLP-1 agonist[1] |
| Main uses | Type 2 diabetes, obesity[1] |
| Side effects | Nausea, vomiting, diarrhea, abdominal pain, constipation, diabetic ketoacidosis, low blood sugar, pancreatitis[2][1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Subcutaneous, by mouth[1] |
| Duration of action | 63.6 h |
| Typical dose | 0.25 to 1 mg SC per week[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618008 |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 89% |
| Metabolism | Proteolysis |
| Elimination half-life | 1 week |
| Excretion | Urine and faeces |
| Chemical and physical data | |
| Formula | C187H291N45O59 |
| Molar mass | 4113.641 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Semaglutide, sold under the brand names Ozempic among others, is a medication used to treat type 2 diabetes and obesity.[1] It is less preferred to metformin, though they may be used together.[1][2] It improves blood sugar control, decreases the risk of heart disease, and decreases the risk of kidney problems.[1] It is used by mouth or by injection under the skin.[1]
Common side effects including nausea, vomiting, diarrhea, abdominal pain, and constipation.[1] Serious side effects may include diabetic ketoacidosis, low blood sugar, and pancreatitis.[2] There are concerns that use during pregnancy may harm the baby and use when breastfeeding is not recommended.[1] It works like human glucagon-like peptide-1 (GLP-1) and increases insulin release, decreases glucagon release, and slows stomach emptying.[2]
Semaglutide was approved for medical use in the United States in 2017.[1] It was developed by Novo Nordisk.[1] It was the first GLP-1 that can be taken by mouth.[6] In the United Kingdom 2 mg for injection costs the NHS about £73 as of 2020.[2] This amount in the United States costs about 850 USD as of 2021.[7]
Medical uses
Semaglutide is used to treat type 2 diabetes.[1]
For obesity it results in about 7 to 13% more weight loss (6 to 12 kg) than a placebo.[8] Though weight is regained if stopped.[8]
Dosage
By mouth, the typical initial dose is 3 mg once per day for the first month.[1] This may than be increased to 7 mg daily and 14 mg daily.[1] Once people are at 14 mg by mouth once a day they may be switched to 0.25 mg once per week for 4 weeks, which is than increased to 0.5 and potentially 1 mg per week.[1] It may also be started as 0.25 mg SC once per week.[2]
For obesity it is used at a dose of 2.4 mg once per week by injection.[8]
It is prepared for subcutaneous injection and is available in prefilled pen. It is recommended for once-weekly injection.[9]
Side effects
Side effects including nausea, vomiting, diarrhea, abdominal pain, and constipation may occur. In people with heart problems, it can cause damage to the back of the eye (retinopathy).[10] Side effects include medullary thyroid cancer, kidney problems, diabetic retinopathy, allergic reactions, low blood sugar, and pancreatitis.[6]
Mechanism of action
Semaglutide is a glucagon-like peptide-1 receptor agonist. It increases the production of insulin, a hormone that lowers the blood sugar level.[11] It also appears to enhance growth of β cells in the pancreas, which are the sites of insulin production.[12] On the other hand it inhibits glucagon, which is a hormone that increases blood sugar. It additionally reduces food intake by lowering appetite and slows down digestion in the stomach.[10] In this way it works in body fat reduction.[9]
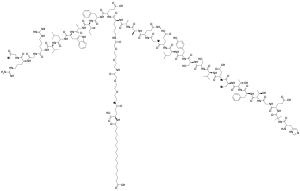
Structure
Semaglutide is chemically similar to human glucagon-like peptide-1 (GLP-1), with 94% similarity. The only differences are two amino acid substitutions at positions 8 and 34, where alanine and lysine are replaced by 2-aminoisobutyric acid and arginine respectively.[13] Amino acid substitution at position 8 prevents chemical breakdown by an enzyme dipeptidyl peptidase-4. In addition, lysine at position 26 is in its derivative form (acylated with stearic diacid). Acylation with a spacer and C-18 fatty diacid chain increases the drug binding to blood protein (albumin), which enables longer presence in the blood circulation.[14] Its half-life in the blood is about 7 days (165–184 hours), therefore, once-weekly injection is enough.[12]
History
Semaglutide was developed in 2012,[15] by a team of researchers at Novo Nordisk as a longer-acting alternative to liraglutide.[16] It was given the brand name Ozempic. Clinical trials were started in 2015, and phase III was completed in 2016.[17]
Researchers at the University of Leeds and Novo Nordisk reported in 2017, that it can also be used for the treatment of obesity.[18] It reduces hunger, food craving and body fat.[19] A Phase 3 Randomized Controlled Trial found that once-weekly injection of 2.4 mg of the drug resulted in an average change of −14.9% body weight at 68 weeks compared to −2.4% for the placebo.[20]
The US FDA New Drug Application (NDA) was filed in December 2016, and in October 2017, the FDA Advisory Committee voted 16–0 in favor.[21] Approval was announced in December 2017 for the injectable version.[22] It can be used as both injection-type or oral-type drug.[23] The marketing authorization in the European Union was granted in February 2018.[4][24] The Japanese Ministry of Health, Labour and Welfare announced approval on 23 March 2018.[25] Health Canada issued approval on 4 January 2018.[26] Semaglutide was approved for medical use in Australia in August 2019.[27]
A version which is taken by mouth (Rybelsus) was approved for medical use in the United States in September 2019,[28] and in the European Union in April 2020.[5]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 "Semaglutide Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 16 January 2021. Retrieved 2 April 2021.
- 1 2 3 4 5 6 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 738. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ↑ "Semaglutide Use During Pregnancy". Drugs.com. Archived from the original on 17 April 2021. Retrieved 16 April 2021.
- 1 2 "Ozempic EPAR". European Medicines Agency (EMA). Archived from the original on 25 October 2020. Retrieved 26 September 2020.
- 1 2 "Rybelsus EPAR". European Medicines Agency (EMA). 29 January 2020. Archived from the original on 14 August 2020. Retrieved 26 September 2020.
- 1 2 "FDA approves first oral GLP-1 treatment for type 2 diabetes" (Press release). FDA. 20 September 2019. Archived from the original on 23 September 2019. Retrieved 20 September 2019.
- ↑ "Ozempic Prices, Coupons & Savings Tips". GoodRx. Archived from the original on 5 September 2021. Retrieved 16 April 2021.
- 1 2 3 Ton, Joey (11 January 2022). "#306 Top 5 Tools for Practice of 2021". CFPCLearn. Archived from the original on 1 July 2023. Retrieved 14 June 2023.
- 1 2 Dhillon S (February 2018). "Semaglutide: First Global Approval". Drugs. 78 (2): 275–284. doi:10.1007/s40265-018-0871-0. PMID 29363040. S2CID 46851453.
- 1 2 Doggrell SA (March 2018). "Sgemaglutide in type 2 diabetes - is it the best glucagon-like peptide 1 receptor agonist (GLP-1R agonist)?" (PDF). Expert Opinion on Drug Metabolism & Toxicology. 14 (3): 371–377. doi:10.1080/17425255.2018.1441286. PMID 29439603. S2CID 3421553. Archived (PDF) from the original on 2020-05-05. Retrieved 2019-12-12.
- ↑ Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. (November 2016). "Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes". The New England Journal of Medicine. 375 (19): 1834–1844. doi:10.1056/NEJMoa1607141. PMID 27633186. Archived from the original on 2021-02-13. Retrieved 2020-09-05.
- 1 2 Goldenberg RM, Steen O (March 2019). "Semaglutide: Review and Place in Therapy for Adults With Type 2 Diabetes". Canadian Journal of Diabetes. 43 (2): 136–145. doi:10.1016/j.jcjd.2018.05.008. PMID 30195966.
- ↑ Lau J, Bloch P, Schäffer L, Pettersson I, Spetzler J, Kofoed J, et al. (September 2015). "Discovery of the Once-Weekly Glucagon-Like Peptide-1 (GLP-1) Analogue Semaglutide". Journal of Medicinal Chemistry. 58 (18): 7370–80. doi:10.1021/acs.jmedchem.5b00726. PMID 26308095.
- ↑ Gotfredsen CF, Mølck AM, Thorup I, Nyborg NC, Salanti Z, Knudsen LB, Larsen MO (July 2014). "The human GLP-1 analogs liraglutide and semaglutide: absence of histopathological effects on the pancreas in nonhuman primates" (PDF). Diabetes. 63 (7): 2486–97. doi:10.2337/db13-1087. PMID 24608440. S2CID 35102048. Archived (PDF) from the original on 2017-09-21. Retrieved 2018-08-22.
- ↑ "Abstracts of the 48th EASD (European Association for the Study of Diabetes) Annual Meeting of the European Association for the Study of Diabetes. October 1-5, 2012. Berlin, Germany". Diabetologia. 55 Suppl 1 (S1): S7-537. October 2012. doi:10.1007/s00125-012-2688-9. PMID 22918257.
- ↑ Kalra S, Gupta Y (July 2015). "Once-weekly glucagon-like peptide 1 receptor agonists". JPMA. The Journal of the Pakistan Medical Association. 65 (7): 796–8. PMID 26160096.
- ↑ Clinical trial number NCT02648204 for "Efficacy and Safety of Semaglutide Versus Dulaglutide as add-on to Metformin in Subjects With Type 2 Diabetes" at ClinicalTrials.gov
- ↑ Blundell J, Finlayson G, Axelsen M, Flint A, Gibbons C, Kvist T, Hjerpsted JB (September 2017). "Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity". Diabetes, Obesity & Metabolism. 19 (9): 1242–1251. doi:10.1111/dom.12932. PMC 5573908. PMID 28266779.
- ↑ "Drug can dramatically reduce weight of people with obesity". ScienceDaily. 23 October 2017. Archived from the original on 15 July 2019. Retrieved 24 October 2017.
- ↑ Wilding, John P.H.; Batterham, Rachel L.; Calanna, Salvatore; Davies, Melanie; Van Gaal, Luc F.; Lingvay, Ildiko; McGowan, Barbara M.; Rosenstock, Julio; Tran, Marie T.D.; Wadden, Thomas A.; Wharton, Sean; Yokote, Koutaro; Zeuthen, Niels; Kushner, Robert F. (10 February 2021). "Once-Weekly Semaglutide in Adults with Overweight or Obesity". New England Journal of Medicine: NEJMoa2032183. doi:10.1056/NEJMoa2032183.
- ↑ "Development Status and FDA Approval Process for semaglutide". Drugs.com. 2017. Archived from the original on 24 October 2017. Retrieved 24 October 2017.
- ↑ "Ozempic (semaglutide) Injection". U.S. Food and Drug Administration (FDA). 16 January 2018. Archived from the original on 1 March 2021. Retrieved 26 September 2020.
- ↑ Davies M, Pieber TR, Hartoft-Nielsen ML, Hansen OK, Jabbour S, Rosenstock J (October 2017). "Effect of Oral Semaglutide Compared With Placebo and Subcutaneous Semaglutide on Glycemic Control in Patients With Type 2 Diabetes: A Randomized Clinical Trial". JAMA. 318 (15): 1460–1470. doi:10.1001/jama.2017.14752. PMC 5817971. PMID 29049653.
- ↑ "Novo Nordisk A/S: Ozempic (semaglutide) approved in the EU for the treatment of type 2 diabetes" (Press release). Novo Nordisk A/S. 9 February 2018. Archived from the original on 2019-04-02. Retrieved 2018-08-19 – via GlobeNewswire.
- ↑ "Ozempic approved in Japan for the treatment of type 2 diabetes" (Press release). Novo Nordisk A/S. 23 March 2018. Archived from the original on 2 April 2019. Retrieved 2 April 2019 – via GlobeNewswire.
- ↑ "Regulatory Decision Summary – Ozempic". Health Canada. Archived from the original on 17 May 2019. Retrieved 2 April 2019.
- ↑ "Summary for ARTG Entry:315107 Ozempic 1 mg semaglutide (rys) 1.34 mg/mL solution for injection pre-filled pen" (PDF). Therapeutic Goods Administration (TGA). Retrieved 26 September 2020.
{{cite web}}: CS1 maint: url-status (link) - ↑ "Drug Approval Package: Rybelsus". U.S. Food and Drug Administration (FDA). 10 June 2020. Archived from the original on 2 November 2020. Retrieved 26 September 2020.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- "Semaglutide". MedlinePlus. Archived from the original on 2020-10-18. Retrieved 2020-09-27.