ACT-462206
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| Drug class | Orexin receptor antagonist |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
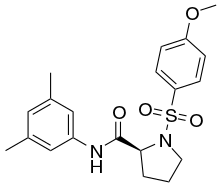
| Formula | C20H24N2O4S |
| Molar mass | 388.48 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
ACT-462206 is a dual orexin receptor antagonist (IC50 for OX1 = 60nM, OX2 = 11nM) which has been investigated for the treatment of insomnia. In human trials, ACT-462206 produced dose-dependent sedative effects and was generally well tolerated, with residual sleepiness and headache being the most common adverse events.[1]
Pharmacology
In humans, the sedative effects of ACT-462206 began 45 minutes after oral administration, and dissispated within 8 hours - consistent with a favorable pharmacodynamic profile for the treatment of insomnia. However, the pharmacokinetic profile of ACT-462206 diverges significantly from what would be expected based on behavioral effects. Elevated plasma concentrations of ACT-462206 are sustained for over 24–36 hours after administration - despite behvioral measures of sedation disappearing within 8 hours of administration.[1]
References
- 1 2 Hoch M, van Gorsel H, van Gerven J, Dingemanse J (September 2014). "Entry-into-humans study with ACT-462206, a novel dual orexin receptor antagonist, comparing its pharmacodynamics with almorexant". Journal of Clinical Pharmacology. 54 (9): 979–986. doi:10.1002/jcph.297. PMID 24691844. S2CID 40714628.