ACT-539313
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| Drug class | Orexin receptor antagonist |
| Pharmacokinetic data | |
| Elimination half-life | 3.3–6.5 hours[1][2] |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
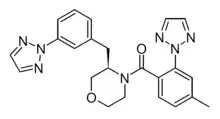
| Formula | C23H23N7O2 |
| Molar mass | 429.484 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
ACT-539313 is an orexin antagonist medication which is under development for the treatment of binge eating disorder and was previously under development for the treatment of anxiety disorders.[4][5][6][7][1][2] It is an orally active small-molecule compound with an elimination half-life of 3.3 to 6.5 hours and acts as a selective orexin OX1 receptor antagonist (1-SORA).[4][1][2] As of May 2022, the drug is in phase 2 clinical trials for binge eating disorder.[4] Following negative efficacy results of a phase 2 trial of ACT-539313 for binge eating disorder, Idorsia (the developer of ACT-539313) signaled in May 2022 that it would not pursue further development of the drug for this indication.[8]
References
- 1 2 3 4 Kaufmann P, Ort M, Golor G, Kornberger R, Dingemanse J (July 2020). "First-in-human study with ACT-539313, a novel selective orexin-1 receptor antagonist". British Journal of Clinical Pharmacology. 86 (7): 1377–1386. doi:10.1111/bcp.14251. PMC 7319015. PMID 32067262.
ACT-539313 ((4-methyl-2-[1,2,3]triazol-2-yl-phenyl)-[(R)- 3-(3-[1,2,3]triazol-2-yl-benzyl)-morpholin-4-yl]-methanone) is an orally active, reversible, selective OX1 receptor antagonist (1-SORA) that readily crosses the blood–brain barrier.
- 1 2 3 Kaufmann P, Ort M, Golor G, Kornberger R, Dingemanse J (June 2021). "Multiple-dose clinical pharmacology of the selective orexin-1 receptor antagonist ACT-539313". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 108: 110166. doi:10.1016/j.pnpbp.2020.110166. PMID 33159976. S2CID 226248763.
- ↑ Dale NC, Hoyer D, Jacobson LH, Pfleger KD, Johnstone EK (2022). "Orexin Signaling: A Complex, Multifaceted Process". Frontiers in Cellular Neuroscience. 16: 812359. doi:10.3389/fncel.2022.812359. PMC 9044999. PMID 35496914.
- 1 2 3 "ACT 539313 - AdisInsight".
- ↑ Jacobson LH, Hoyer D, de Lecea L (May 2022). "Hypocretins (orexins): The ultimate translational neuropeptides". Journal of Internal Medicine. 291 (5): 533–556. doi:10.1111/joim.13406. PMID 35043499. S2CID 248119793.
- ↑ Yaeger JD, Krupp KT, Gale JJ, Summers CH (December 2020). "Counterbalanced microcircuits for Orx1 and Orx2 regulation of stress reactivity". Medicine in Drug Discovery. 8: 100059. doi:10.1016/j.medidd.2020.100059. ISSN 2590-0986. S2CID 224861235.
- ↑ Caldirola D, Alciati A, Cuniberti F, Perna G (2021). "Experimental Drugs for Panic Disorder: An Updated Systematic Review". Journal of Experimental Pharmacology. 13: 441–459. doi:10.2147/JEP.S261403. PMC 8055642. PMID 33889031.
- ↑ "Just months after its first FDA approval, Idorsia dumps binge-eating drug candidate after PhII fail".
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.