Suntinorexton
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
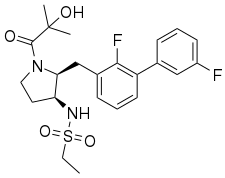
| Formula | C23H28F2N2O4S |
| Molar mass | 466.54 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Suntinorexton (INN) is an experimental orexin receptor agonist..[1] It acts as a selective agonist of the orexin OX2 receptor and was described in 2019 in a patent by Takeda Pharmaceutical Company.[2]
See also
- Orexin receptor § Agonists
- List of investigational sleep drugs § Orexin receptor agonists
References
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN)" (PDF). WHO Drug Information. 34 (1): 93–269. 2020.
Proposed INN: List 123
- ↑ WO application 2019027058, Kajita Y, Mikami S, Miyanohana Y, Koike T, Daini M, Oyabu N, Ogino M, Takeuchi K, Ito Y, Tokunaga N, Sugimoto T, Miyazaki T, Oda T, Hoashi Y, Hattori Y, Imamura K, "Heterocyclic compound and use therof", published 2019-02-07, assigned to Takeda Pharmaceutical Company.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.