Danavorexton
 | |
| Clinical data | |
|---|---|
| Other names | TAK-925 |
| Routes of administration | Intravenous |
| Drug class | Orexin receptor agonist |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
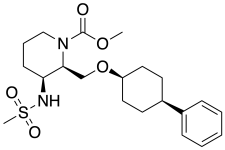
| Formula | C21H32N2O5S |
| Molar mass | 424.56 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Danavorexton (developmental code name TAK-925) is a selective orexin 2 receptor agonist. As of March 2021, danavorexton is under investigation for the treatment of narcolepsy, idiopathic hypersomnia, and sleep apnea.[1][2]
See also
- Orexin receptor § Agonists
- List of investigational sleep drugs § Orexin receptor agonists
References
- ↑ "Danavorexton - Takeda". Adis Insight. Springer Nature Switzerland AG. Retrieved 7 March 2021.
- ↑ Evans R, Tanaka S, Tanaka S, Touno S, Shimizu K, Sakui S, et al. (December 2019). "A Phase 1 single ascending dose study of a novel orexin 2 receptor agonist, TAK-925, in healthy volunteers (HV) and subjects with narcolepsy type 1 (NT1) to assess safety, tolerability, pharmacokinetics, and pharmacodynamic outcomes". Sleep Medicine. 64: S105–S106. doi:10.1016/j.sleep.2019.11.290.
External links
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.