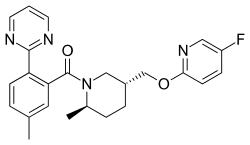
Filorexant
 | |
| Clinical data | |
|---|---|
| Routes of administration | By mouth |
| Drug class | Orexin antagonist |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.203.042 |
| Chemical and physical data | |
| Formula | C24H25FN4O2 |
| Molar mass | 420.488 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Filorexant (INN, USAN) (code name MK-6096) is an orexin antagonist which is or was under development by Merck for the treatment of insomnia.[1] It is a dual antagonist of the OX1 and OX2 receptors.[2][3] As of March 2014, filorexant has completed phase II clinical trials.[4] It was also investigated as a migraine prophylaxis, but was not found effective,[5] and in major depressive disorder and painful diabetic neuropathy.[6] As of May 2015, filorexant is no longer listed on Merck's online development pipeline.[7]
See also
References
- ↑ Hoyer D, Jacobson LH (December 2013). "Orexin in sleep, addiction and more: is the perfect insomnia drug at hand?". Neuropeptides. 47 (6): 477–88. doi:10.1016/j.npep.2013.10.009. PMID 24215799. S2CID 6402764.
- ↑ Winrow CJ, Gotter AL, Cox CD, et al. (February 2012). "Pharmacological characterization of MK-6096 - a dual orexin receptor antagonist for insomnia". Neuropharmacology. 62 (2): 978–87. doi:10.1016/j.neuropharm.2011.10.003. PMID 22019562. S2CID 35304627.
- ↑ Peroutka SJ (January 2014). "Clinical trials update. 2013: year in review". Headache. 54 (1): 189–94. doi:10.1111/head.12267. PMID 24400767. S2CID 28141555.
- ↑ Cooper CK (March 2014). "Orexin Receptor Antagonists: Novel Hypnotic Agents". Ment. Health Clin. 4 (2): 64. doi:10.9740/mhc.n190094. ISSN 2168-9709.
- ↑ Chabi, A.; Zhang, Y.; Jackson, S.; Cady, R.; Lines, C.; Herring, W. J.; Connor, K. M.; Michelson, D. (Aug 8, 2014). "Randomized controlled trial of the orexin receptor antagonist filorexant for migraine prophylaxis". Cephalalgia. 35 (5): 379–88. doi:10.1177/0333102414544979. PMID 25106663. S2CID 20872932.
- ↑ Michel Alexander Steiner; Christopher J Winrow (11 November 2014). Insomnia and beyond - Exploring the therapeutic potential of orexin receptor antagonists. Frontiers E-books. pp. 3–. ISBN 978-2-88919-330-1.
- ↑ "Merck Pipeline". Merck. 2015. Retrieved 2015-05-14.
External links
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.