Lipolysis
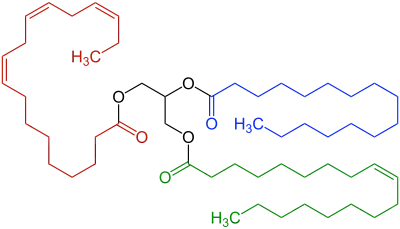
Lipolysis /lɪˈpɒlɪsɪs/ is the metabolic pathway through which lipid triglycerides are hydrolyzed into a glycerol and three fatty acids. It is used to mobilize stored energy during fasting or exercise, and usually occurs in fat adipocytes. The most important regulatory hormone in lipolysis is insulin; lipolysis can only occur when insulin action falls to low levels, as occurs during fasting. Other hormones that affect lipolysis include glucagon,[1] epinephrine, norepinephrine, growth hormone, atrial natriuretic peptide, brain natriuretic peptide, and cortisol.[2]
Mechanisms
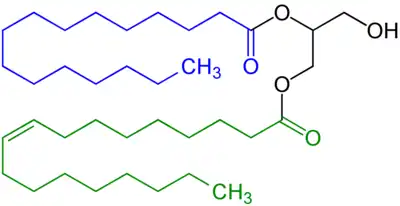
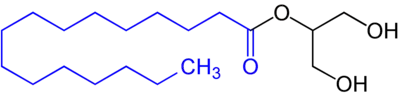
In the body, stores of fat are referred to as adipose tissue. In these areas, intracellular triglycerides are stored in cytoplasmic lipid droplets. When lipases are phosphorylated, they can access lipid droplets and through multiple steps of hydrolysis, breakdown triglycerides into fatty acids and glycerol. Each step of hydrolysis leads to the removal of one fatty acid. The first step and the rate-limiting step of lipolysis is carried out by adipose triglyceride lipase (ATGL). This enzyme catalyzes the hydrolysis of triacylglycerol to diacylglycerol. Subsequently, hormone-sensitive lipase (HSL) catalyzes the hydrolysis of diacylglycerol to monoacylglycerol and monoacylglycerol lipase (MGL) catalyzes the hydrolysis of monoacylglycerol to glycerol.[3]
Perilipin 1A is a key protein regulator of lipolysis in adipose tissue. This lipid droplet-associated protein, when deactivated, will prevent the interaction of lipases with triglycerides in the lipid droplet and grasp the ATGL co-activator, comparative gene identification 58 (CGI-58) (a.k.a. ABHD5). When perilipin 1A is phosphorylated by PKA, it releases CGI-58 and it expedites the docking of phosphorylated lipases to the lipid droplet.[4] CGI-58 can be further phosphorylated by PKA to assist in its dispersal to the cytoplasm. In the cytoplasm, CGI-58 can co-activate ATGL.[5] ATGL activity is also impacted by the negative regulator of lipolysis, G0/G1 switch gene 2 (G0S2). When expressed, G0S2 acts as a competitive inhibitor in the binding of CGI-58.[6] Fat-specific protein 27 (FSP-27) (a.k.a. CIDEC) is also a negative regulator of lipolysis. FSP-27 expression is negatively correlated with ATGL mRNA levels.[7]
Regulation

The cAMP activates protein kinases, which phosphorylate and thus activate hormone-sensitive lipases in the adipocyte.
These lipases cleave free fatty acids from their attachment to glycerol in the lipid droplet of the adipocyte.
The free fatty acids and glycerol are then released into the blood.
The activity of hormone sensitive lipase is regulated by the circulating hormones insulin, glucagon, norepinephrine, and epinephrine.
Lipolysis can be regulated through cAMP's binding and activation of protein kinase A (PKA). PKA can phosphorylate lipases, perilipin 1A, and CGI-58 to increase the rate of lipolysis. Catecholamines bind to 7TM receptors (G protein-coupled receptors) on the adipocyte cell membrane, which activate adenylate cyclase. This results in increased production of cAMP, which activates PKA and leads to an increased rate of lipolysis. Despite glucagon's lipolytic activity (which stimulates PKA as well) in vitro, the role of glucagon in lipolysis in vivo is disputed.[8]
Insulin counter-regulates this increase in lipolysis when it binds to insulin receptors on the adipocyte cell membrane. Insulin receptors activate insulin-like receptor substrates. These substrates activate phosphoinositide 3-kinases (PI-3K) which then phosphorylate protein kinase B (PKB) (a.k.a. Akt). PKB subsequently phosphorylates phosphodiesterase 3B (PD3B), which then converts the cAMP produced by adenylate cyclase into 5'AMP. The resulting insulin induced reduction in cAMP levels decreases the lipolysis rate.[9]
Insulin also acts in the brain at the mediobasal hypothalamus. There, it suppresses lipolysis and decreases sympathetic nervous outflow to the fatty part of the brain matter.[10] The regulation of this process involves interactions between insulin receptors and gangliosides present in the neuronal cell membrane.[11]
In blood
Triglycerides are transported through the blood to appropriate tissues (adipose, muscle, etc.) by lipoproteins such as Very-Low-Density-Lipoproteins (VLDL). Triglycerides present on the VLDL undergo lipolysis by the cellular lipases of target tissues, which yields glycerol and free fatty acids. Free fatty acids released into the blood are then available for cellular uptake.[12] Free fatty acids not immediately taken up by cells may bind to albumin for transport to surrounding tissues that require energy. Serum albumin is the major carrier of free fatty acids in the blood.[13]
The glycerol also enters the bloodstream and is absorbed by the liver or kidney where it is converted to glycerol 3-phosphate by the enzyme glycerol kinase. Hepatic glycerol 3-phosphate is converted mostly into dihydroxyacetonephosphate (DHAP) and then glyceraldehyde 3-phosphate (GA3P) to rejoin the glycolysis and gluconeogenesis pathway.
Lipogenesis
While lipolysis is triglyceride hydrolysis (the process by which triglycerides are broken down), esterification is the process by which triglycerides are formed. Esterification and lipolysis are, in essence, reversals of one another.[14]
Medical procedures
Physical lipolysis involves destruction of fat cells containing the fat droplets and can be used as part of cosmetic body contouring procedures. Currently there are four main non-invasive body contouring techniques in aesthetic medicine for reducing localized subcutaneous adipose tissue in addition to the standard minimally invasive liposuction: low-level laser therapy (LLLT), cryolipolysis, radio frequency (RF) and high-intensity focused ultrasound (HIFU).[15][16] However, they are less effective with shorter lasting benefits and can remove significantly smaller amounts of fat compared to traditional surgical liposuction or lipectomy. However, future drug developments can be potentially combined with smaller procedures to augment the final result.
References
- ↑ Duncan, Robin E.; Ahmadian, Maryam; Jaworski, Kathy; Sarkadi-Nagy, Eszter; Sul, Hei Sook (August 2007). "Regulation of Lipolysis in Adipocytes". Annual Review of Nutrition. 27 (1): 79–101. doi:10.1146/annurev.nutr.27.061406.093734. PMC 2885771. PMID 17313320.
- ↑ Nielsen, TS; Jessen, N; Jørgensen, JO; Møller, N; Lund, S (June 2014). "Dissecting adipose tissue lipolysis: molecular regulation and implications for metabolic disease". Journal of Molecular Endocrinology. 52 (3): R199–222. doi:10.1530/JME-13-0277. PMID 24577718.
- ↑ Frühbeck, G; Méndez-Giménez, L; Fernández-Formoso, JA; Fernández, S; Rodríguez, A (June 2014). "Regulation of adipocyte lipolysis". Nutrition Research Reviews. 27 (1): 63–93. doi:10.1017/S095442241400002X. PMID 24872083.
- ↑ Itabe, H; Yamaguchi, T; Nimura, S; Sasabe, N (28 April 2017). "Perilipins: a diversity of intracellular lipid droplet proteins". Lipids in Health and Disease. 16 (1): 83. doi:10.1186/s12944-017-0473-y. PMC 5410086. PMID 28454542.
- ↑ Sahu-Osen, A; Montero-Moran, G; Schittmayer, M; Fritz, K; Dinh, A; Chang, YF; McMahon, D; Boeszoermenyi, A; Cornaciu, I; Russell, D; Oberer, M; Carman, GM; Birner-Gruenberger, R; Brasaemle, DL (January 2015). "CGI-58/ABHD5 is phosphorylated on Ser239 by protein kinase A: control of subcellular localization". Journal of Lipid Research. 56 (1): 109–21. doi:10.1194/jlr.M055004. PMC 4274058. PMID 25421061.
- ↑ Cornaciu, I; Boeszoermenyi, A; Lindermuth, H; Nagy, HM; Cerk, IK; Ebner, C; Salzburger, B; Gruber, A; Schweiger, M; Zechner, R; Lass, A; Zimmermann, R; Oberer, M (2011). "The minimal domain of adipose triglyceride lipase (ATGL) ranges until leucine 254 and can be activated and inhibited by CGI-58 and G0S2, respectively". PLOS ONE. 6 (10): e26349. Bibcode:2011PLoSO...626349C. doi:10.1371/journal.pone.0026349. PMC 3198459. PMID 22039468.
- ↑ Singh, M; Kaur, R; Lee, MJ; Pickering, RT; Sharma, VM; Puri, V; Kandror, KV (23 May 2014). "Fat-specific protein 27 inhibits lipolysis by facilitating the inhibitory effect of transcription factor Egr1 on transcription of adipose triglyceride lipase". The Journal of Biological Chemistry. 289 (21): 14481–7. doi:10.1074/jbc.C114.563080. PMC 4031504. PMID 24742676.
- ↑ Schmitz, Ole; Christiansen, Jens Sandahl; Jensen, Michael D.; Møller, Niels; Gravholt, Claus Højbjerg (1 May 2001). "Physiological Levels of Glucagon Do Not Influence Lipolysis in Abdominal Adipose Tissue as Assessed by Microdialysis". The Journal of Clinical Endocrinology & Metabolism. 86 (5): 2085–2089. doi:10.1210/jcem.86.5.7460. ISSN 0021-972X. PMID 11344211.
- ↑ Jocken, JW; Blaak, EE (23 May 2008). "Catecholamine-induced lipolysis in adipose tissue and skeletal muscle in obesity". Physiology & Behavior. 94 (2): 219–30. doi:10.1016/j.physbeh.2008.01.002. PMID 18262211. S2CID 28173901.
- ↑ Scherer T.; O'Hare J.; Diggs-Andrews K.; Schweizer M.; Check B.; Lindner C.; et al. (1 February 2011). "Brain Insulin Controls Adipose Tissue Lipolysis and Lipogenesis". Cell Metabolism. 13 (2): 183–194. doi:10.1016/j.cmet.2011.01.008. PMC 3061443. PMID 21284985.
- ↑ Herzer, Silke; Meldner, Sascha; Gröne, Hermann-Josef; Nordström, Viola (1 October 2015). "Fasting-Induced Lipolysis and Hypothalamic Insulin Signaling Are Regulated by Neuronal Glucosylceramide Synthase" (PDF). Diabetes. 64 (10): 3363–3376. doi:10.2337/db14-1726. ISSN 0012-1797. PMID 26038579.
- ↑ King, Michael W. "Oxidation of Fatty Acids". Archived from the original on 14 January 2016. Retrieved 9 April 2012.
- ↑ Tom Brody, Nutritional Biochemistry, (Academic Press, 2nd edition 1999), 215-216. ISBN 0121348369
- ↑ Baldwin, Kenneth David Sutherland; Brooks, George H.; Fahey, Thomas D. (2005). Exercise physiology: human bioenergetics and its applications. New York: McGraw-Hill. ISBN 978-0-07-255642-1.
- ↑ Kennedy, J.; Verne, S.; Griffith, R.; Falto-Aizpurua, L.; Nouri, K. (2015). "Non-invasive subcutaneous fat reduction: A review". Journal of the European Academy of Dermatology and Venereology. 29 (9): 1679–88. doi:10.1111/jdv.12994. PMID 25664493. S2CID 40858507.
- ↑ Mulholland, R. Stephen; Paul, Malcolm D.; Chalfoun, Charbel (2011). "Noninvasive Body Contouring with Radiofrequency, Ultrasound, Cryolipolysis, and Low-Level Laser Therapy". Clinics in Plastic Surgery. 38 (3): 503–20, vii–iii. doi:10.1016/j.cps.2011.05.002. PMID 21824546.
External links
- Lipolysis at the US National Library of Medicine Medical Subject Headings (MeSH)
.svg.png.webp)